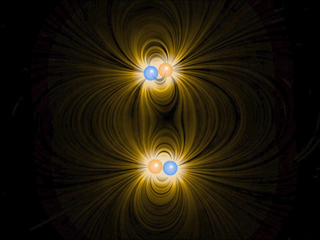
| DESCRIPTION:
We show the interaction of four charges of equal mass.
Two of the charges are positively charged and two of
the charges are negatively charged, and all have the
same magnitude of charge. The particles interact via
the Coulomb force. We also introduce a quantum-mechanical
"Pauli" force, which is always repulsive and
becomes very important at small distances, but is negligible
at large distances. This critical distance is about
the radius of the spheres shown in the animation. This
"Pauli" force is quantum mechanical in origin,
and keeps the charges from collapsing into a point (i.e.,
it keeps a negative particle and a positive particle
from sitting exactly on top of one another). Additionally,
the motion of the particles is damped by a term proportional
to their velocity, allowing them to "settle down"
into stable (or meta-stable) states.
When these charges are allowed to evolve from the initial
state, the first thing that happens (very quickly) is
that the charges pair off into dipoles. This is a rapid
process because the Coulomb attraction between unbalanced
charges is very large. This process is called "ionic
binding", and is responsible for the intermolecular
forces in ordinary table salt, NaCl. After the dipoles
form, there is still an interaction between neighboring
dipoles, but this is a much weaker interaction because
the electric field of the dipoles falls off much faster
than that of a single charge. This is because the net
charge of the dipole is zero.
Although in principle the dipole-dipole interaction
can be either repulsive or attractive, in practice there
is a torque that rotates the dipoles so that the dipole-dipole
force is attractive. This dipole-dipole attraction eventually
brings the two dipoles together in a bound state. The
force of attraction between two dipoles is termed a
"van der Waals" force, and is responsible
for the intermolecular forces that bind the molecules
of some substances into a solid.
|
|