
April-June 2000 Issue
Inside Engine Cylinders: Cleaner Walls for Lower Emissions, Higher Efficiency

April-June 2000 Issue
Inside Engine Cylinders: Cleaner Walls for Lower Emissions,
Higher Efficiency
![]() hen fuel and air burn inside an internal combustion engine, deposits form
on the walls inside the cylinders--a real headache for engine designers. The
coated walls trap heat inside the cylinder, and the increased temperatures
that result affect emissions and impede changes that could increase fuel efficiency.
A new Energy Laboratory model may help designers bring about changes in fuels
or engines that will discourage deposit formation. The numerical model simulates
the chemical reactions that produce deposit "precursors"; the processes
that carry those precursors to the wall; and the condensation, evaporation,
and chemical interactions that occur at the wall. Using models of precursor-forming
reactions designed to bracket real-world engine and fuel conditions, the simulation
generates results that are consistent with deposit growth observed experimentally.
The results also suggest that the only way to stop precursors from landing
on the wall is by preventing their condensation. Raising the wall temperature
would prevent condensation but would defeat the overall goal of keeping cylinder
temperatures down. The MIT student performing the research is now gathering
precise data on precursors and their behavior by using the model plus measurements
taken in a specially designed low-pressure flame. With sufficient data, the
model may help designers identify practical strategies for keeping the precursors
from forming in the first place.
hen fuel and air burn inside an internal combustion engine, deposits form
on the walls inside the cylinders--a real headache for engine designers. The
coated walls trap heat inside the cylinder, and the increased temperatures
that result affect emissions and impede changes that could increase fuel efficiency.
A new Energy Laboratory model may help designers bring about changes in fuels
or engines that will discourage deposit formation. The numerical model simulates
the chemical reactions that produce deposit "precursors"; the processes
that carry those precursors to the wall; and the condensation, evaporation,
and chemical interactions that occur at the wall. Using models of precursor-forming
reactions designed to bracket real-world engine and fuel conditions, the simulation
generates results that are consistent with deposit growth observed experimentally.
The results also suggest that the only way to stop precursors from landing
on the wall is by preventing their condensation. Raising the wall temperature
would prevent condensation but would defeat the overall goal of keeping cylinder
temperatures down. The MIT student performing the research is now gathering
precise data on precursors and their behavior by using the model plus measurements
taken in a specially designed low-pressure flame. With sufficient data, the
model may help designers identify practical strategies for keeping the precursors
from forming in the first place.
Surfaces inside internal combustion engines are dirty. Wherever fuel or fuel-and-air mixture comes into contact with a surface, deposits form. Fuel additives help keep some areas clean, but deposits persist on surfaces inside the combustion chamber. And those deposits have significant effects. The deposit layer on the cylinder wall prevents the coolant that runs through the engine from bringing down the temperature inside the cylinder. Due to the increased temperatures, parts of the fuel-air mixture may ignite before the flame front reaches them, causing the engine to "knock." Another effect of the raised temperatures is increased production of pollutants such as nitrogen oxides inside the engine cylinder. Engineers minimize both of those problems by keeping the compression ratio of the engine relatively low--an approach that brings down fuel efficiency.
While engine experts understand the problems caused by combustion chamber deposits, they do not fully understand how those deposits form. As a result, finding ways to change fuels or engines to prevent deposit formation is difficult, at best. To help in that effort, Mr. Christopher J. O達rien has spent the past two years investigating chemical and physical processes inside engine cylinders. The work was formulated under the direction of Professor Simone Hochgreb and now involves several colleagues in the Sloan Automotive Laboratory, which is directed by Professor John B. Heywood. Although this work focuses on wall deposits inside gasoline engines, the approach and analytical techniques involved are similar to those used by Professors William H. Green and Paul I. Barton in analyzing the performance of diesel engines (see previous article). The two research groups are planning closer collaboration in the future.
The figure below shows the many processes involved in the buildup of deposits inside cylinders. The dashed line near the top of the figure shows how the temperature inside the cylinder varies with distance from the wall as the flame approaches. Well away from the wall, the temperature is high and normal combustion chemistry proceeds. The lightweight molecules that form are highly volatile, that is, they evaporate readily. They are therefore not prone to condensing on surfaces (should they encounter any). However, as the flame nears the wall, it cools; and in this moderate-temperature region, partially burned fuel components turn into heavier, less volatile species (step 1 in the figure). Low-volatility species tend to condense readily and evaporate with difficulty, so if they land on the cold wall they are likely to stay there. They are therefore the deposit precursors of concern.
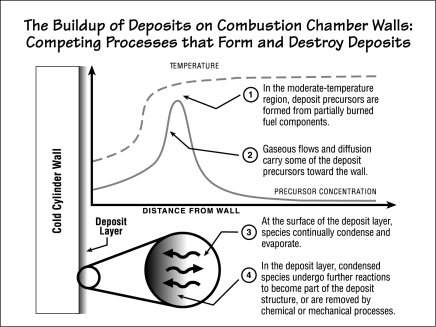
Gaseous flows and diffusion inside the cylinder move the newly formed deposit precursors both toward the wall and away from it (step 2). Some of them reach the cylinder wall, where various processes add to and deplete the deposit. At the surface of the deposit layer, precursors condense and evaporate as temperatures and pressures inside the cylinder change (step 3). Within the deposit, condensed species chemically react to become part of the deposit; and species already in the deposit are removed by chemical processes such as oxidation and gasification and by mechanical processes such as abrasion and flaking (step 4). After a few hundred hours of engine operation, the net effects of the formation and removal processes yield a deposit with a fairly stable thickness.
Within this array of complicated processes Mr. O達rien sees several possibilities for interfering with the buildup of deposits. Keeping the precursors from diffusing to the wall after they form--step 2 in the figure--would be extremely difficult. Two other approaches seem more promising. By changing fuel composition or engine operating conditions, designers might be able to prevent the precursors from forming (step 1), or they might be able to keep them from depositing on the wall (steps 3 and 4).
To investigate those possibilities, Mr. O達rien is working to clarify the processes that take place in the moderate-temperature region of the cylinder close to and at the wall. Developing the needed detailed understanding experimentally would be difficult: in an operating engine, pressures are high and everything is changing rapidly. He therefore began by developing a numerical model that could help in the investigation.
Combustion models exist that describe the behavior of a stationary flame under constant conditions. However, Mr. O達rien needed a model that would describe what happens to a moving flame close to the cylinder wall using complex chemical systems. Based on available models of flame chemistry, he formulated a numerical model that can follow the flame as it travels through the region of interest. Given starting conditions and a description of the fuel, the model calculates chemical reactions, flows, temperature, pressure, and other important variables for a given position of the flame. It then moves forward one time step and, based on outputs from the first calculations, performs the same calculations, yielding information for a subsequent position along the flame痴 travels. At the wall, the model calculates condensation, evaporation, and reactions between the deposit layer and the gases in contact with it. By performing a series of calculations for sequential time steps, the model produces a complete description of what happens as the flame enters at the edge of the area being simulated, propagates across that area, and hits the cold wall.
In theory, the model is capable of simulating all the reactions that take place inside a deposit-producing engine. However, there are several practical problems. A real flame involves hundreds of species and thousands of reactions; running a simulation would require weeks of computer time and would produce masses of data. Therefore, Mr. O達rien tracks only about thirty key species in his model. To that simplified flame chemistry he adds a single reaction by which a selected component in the fuel can form a deposit precursor. But another problem arises. While the chemistry within a hot flame has been experimentally defined fairly well, reaction rates and other key data are not available for the precursor species formed in the moderate-temperature region.
Despite the lack of data, Mr. O達rien has already performed simulations based on a series of reasonable assumptions about the behavior and nature of precursors; and the results have been valuable, both in validating the model and in establishing the relative importance of certain processes. His assumptions concern two variables critical to deposit buildup. The first is reaction rate, that is, how rapidly the precursor forms from the fuel as a function of temperature and other conditions. In different runs, Mr. O達rien assumes reaction rates that range from so slow that none of the precursor forms to so fast that the precursor is abundant. The second variable is the volatility of the precursor that forms. (Again, the lower the volatility, the "stickier" the species at the wall.) In various runs, he assumes a range of volatilities based on physical properties observed experimentally with real fuels. The series of simulations based on the various assumptions generates a range of feasible values for how much of the precursor forms, how much reaches the wall, and how much condenses and forms a deposit.
Certain sets of assumptions describe quite closely the behavior and nature of selected components in real fuels that researchers elsewhere have tested in experimental engines. An opportunity arises, therefore, to assess the performance of the model by comparison to experimental observations. Because experimental equipment and procedures vary so widely, Mr. O達rien focuses on comparing trends. For example, how does the thickness of the deposit vary with fuel concentration or with operating conditions? The simulation results show the same trends as those reported in experiments using comparable materials.
Interestingly, the simulation results also yield unexpected insights into what happens at the wall. Engine experts have a number of theories about how precursors stick. Some believe that they simply condense, like water vapor condensing out of the air. Some believe that various chemical processes are involved. And others are convinced that the surface roughness of the wall is a key factor. Mr. O'Brien痴 analyses suggest that simple condensation is the primary mechanism for deposit formation. Simulations that include only condensation at the wall produce estimated deposit growth rates that match experimental observations.
That outcome is bad news for engine designers. While condensation is well understood, defining a practical strategy to prevent it inside an engine cylinder is difficult. Increasing the wall temperature would work nicely--but the main goal of preventing deposits is to keep temperatures low to inhibit knock and limit nitrogen oxides formation. Since keeping the precursors from reaching the wall and condensing seems unlikely, attention must focus on preventing the precursors from forming in the first place.
The MIT model could provide valuable insight into how to alter fuel composition to keep sticky precursors from forming--if data were available on reaction rates and other details involved in precursor formation. Mr. O達rien is now gathering the needed data and observing deposit behavior using an experimental apparatus that he designed and built during the past year. Rather than trying to replicate engine conditions, he uses a low-pressure, steady-state flame and then turns to the numerical model, which can simulate a wide range of conditions. Thus, based on measurements taken in the simple experimental environment, the model can calculate flows, deposit growth rates, and so on under actual engine conditions.
To simulate the cylinder wall, Mr. O達rien uses a flat copper plate that is water-cooled from below. A mixture of fuel and air blows up through small holes in the plate and ignites above it. A stationary probe gathers samples within the flame. The speed of the fuel-air mixture and the pressure of the vessel determine how far above the plate the flame rests and how much it is cooled by the plate. By moving the plate closer to and farther from the probe, Mr. O達rien can gather samples at different locations within the flame, thereby investigating processes that occur at varying distances from the cylinder wall. Samples are analyzed on-site and also in the Analytical Chemistry Laboratory of the Center for Environmental Health Sciences, which is headed by Dr. Arthur Lafleur and has sophisticated instrumentation and techniques for looking at such materials.
Thus far, Mr. O達rien has performed preliminary experiments with propane and air flames. Although such flames do not create deposits, results have been encouraging. In particular, experimental measurements of concentrations of carbon monoxide, carbon dioxide, and hydrogen at various locations match predictions generated by model simulations under comparable conditions. The next step is to add--one at a time--fuel components such as benzene, toluene, and other species known to cause deposits. Tests with other fuel components may lead to identification of not-yet-recognized precursor species.
Mr. O達rien is far from tackling the complex chemistry of real flames. He will need a detailed data base on all the chemical species and how they interact with each other. For now, he is just trying to establish the building blocks underlying the complex combustion process. But those building blocks may be all the automotive and fuel companies need to design practical strategies for dealing with deposits, especially if the sticky precursors form from a few reactions early in the burning and cooling process. Already Mr. O達rien has provided his model to several automobile and oil companies for review. With additional data on precursors and their behavior, Mr. O達rien hopes that the model will enable engine experts to identify a series of changes in fuels and engine operating conditions that will not only control deposits but also reduce emissions and increase efficiency.
Christopher J. O達rien is a PhD candidate in MIT痴 Department of Mechanical Engineering. Simone Hochgreb was an associate professor mechanical engineering through 1998. She is now at the Combustion Research Facility of the Sandia National Laboratories. John B. Heywood is Sun Jae Professor of Mechanical Engineering and director of the Sloan Automotive Laboratory. Arthur L. Lafleur is associate director of the Center for Environmental Health Sciences. This research was supported by the MIT Engine and Fuels Consortium, which now includes DaimlerChrysler, ExxonMobil, Ford Motor Company, General Motors Corporation, Shell, and Volvo Car Corporation. Publications are forthcoming.