
July-September 2000 Issue
Running Buses on Hydrogen Fuel Cells: Barriers and Opportunities

July-September
2000 Issue
Running Buses on Hydrogen Fuel
Cells: Barriers and Opportunities
![]() ehicles running on fuel cells fed by hydrogen could be ideal environmentally
for crowded cities: they are quiet and clean, emitting none of the air pollutants
that now plague urban areas. And emissions of greenhouse gases could be eliminated
as well if the hydrogen were made using carbon-free sources such as solar power.
But figuring out how to deliver hydrogen to private vehicles is a daunting problem,
given today's fuel handling and storage technologies. Energy Laboratory researchers
have looked at a more manageable application of this technology: in fleets of
buses. They focused on a demonstration in which the Sunline Transit Agency in
Los Angeles will gradually switch its buses from compressed natural gas engines
to fuel cells, powered first by commercially provided liquid hydrogen and subsequently
by compressed hydrogen gas that Sunline itself will manufacture from natural
gas. This commercial experience will help clarify the issues involved in producing,
handling, and storing hydrogen; maintaining and operating vehicles; and providing
a given level of service. The MIT assessment identifies many of the practical
hurdles Sunline must overcome, from setting up its hydrogen fueling station
to retraining its managers and operators. Broader issues include the public's
perception of hydrogen fuel as dangerous; potentially high costs; and still-evolving
safety, zoning, and other regulations. Whether hydrogen will become the clean
transportation fuel of the future remains to be seen. But if all goes as planned,
Sunline's customers will get a first taste of the potential benefits of this
technology: clean, quiet buses that get them where they need to go.
ehicles running on fuel cells fed by hydrogen could be ideal environmentally
for crowded cities: they are quiet and clean, emitting none of the air pollutants
that now plague urban areas. And emissions of greenhouse gases could be eliminated
as well if the hydrogen were made using carbon-free sources such as solar power.
But figuring out how to deliver hydrogen to private vehicles is a daunting problem,
given today's fuel handling and storage technologies. Energy Laboratory researchers
have looked at a more manageable application of this technology: in fleets of
buses. They focused on a demonstration in which the Sunline Transit Agency in
Los Angeles will gradually switch its buses from compressed natural gas engines
to fuel cells, powered first by commercially provided liquid hydrogen and subsequently
by compressed hydrogen gas that Sunline itself will manufacture from natural
gas. This commercial experience will help clarify the issues involved in producing,
handling, and storing hydrogen; maintaining and operating vehicles; and providing
a given level of service. The MIT assessment identifies many of the practical
hurdles Sunline must overcome, from setting up its hydrogen fueling station
to retraining its managers and operators. Broader issues include the public's
perception of hydrogen fuel as dangerous; potentially high costs; and still-evolving
safety, zoning, and other regulations. Whether hydrogen will become the clean
transportation fuel of the future remains to be seen. But if all goes as planned,
Sunline's customers will get a first taste of the potential benefits of this
technology: clean, quiet buses that get them where they need to go.
In the United States, on-road vehicles emit more than a quarter of all domestic carbon dioxide (CO2) emissions as well as large quantities of local emissions including hydrocarbons, nitrogen oxides, and particulates. One way to eliminate those local emissions could be to run cars on fuel cells fed by hydrogen. Combining hydrogen with oxygen in a fuel cell produces electricity, a bit of heat, and water. Unfortunately, pure hydrogen does not occur in nature; so getting hydrogen for fuel requires separating it from plentiful compounds such as natural gas or water. If the hydrogen is made from natural gas, CO2 emissions could drop by up to 25% compared to today's gasoline vehicles. And if it is made from non-carbon sources or using carbon capture and sequestration technologies, CO2 emissions are eliminated. (The main competing "zero-emission vehicle" technology is the electric car, but battery storage of electricity is cumbersome and limits the distance a vehicle can travel between refueling stops.)
Automakers have demonstrated prototype vehicles that run on hydrogen fuel cells, but distributing hydrogen to today's drivers seems an overwhelming problem. Using hydrogen fuel cell technology in fleets of commercial vehicles such as buses might make more sense. A centralized hydrogen fueling station could serve an entire fleet of buses; a single fleet manager could oversee the maintenance of the fleet and supporting facilities; and the service routes for the vehicles could be carefully planned to fit their capabilities.
Given the many unknowns in such an undertaking, real-world testing is a necessity. Graduate student Jane Brydges working with Dr. Elisabeth M. Drake assessed the challenges involved in designing and implementing a fleet demonstration. What practical steps are required to develop and support a system of hydrogen fuel cell buses? And what barriers must be overcome for the fleet test to occur? Answering those questions should shed light on the problems involved in the wider adoption of hydrogen in commercial or even passenger vehicles.
In their assessment, the researchers focused on a demonstration planned by Sunline Transit Agency, a company that operates a fleet of 50 buses in the Coachella Valley near Los Angeles, California. The Los Angeles area is highly motivated to test alternative transportation systems because air pollution is severe, road travel is critical to the city's lifestyle, and clean air regulations are unusually stringent. Within three years, a fraction of all buses must be zero-emissions vehicles, and fuel cell buses carrying their own hydrogen supply will qualify--and will be quiet as well.
In reviewing various demonstrations of alternative fuels, the MIT researchers concluded that one key to success is a company's commitment. Sunline has a strong history of such commitment. In 1994, it replaced its entire aging diesel fleet with compressed natural gas (CNG) vehicles. It bought equipment, retrained its personnel, and worked closely with the community. Operating costs declined, ridership increased, and emissions of local pollutants dropped. Now Sunline's managers are planning to reduce local pollutant emissions still further by switching at least some of their CNG buses to hydrogen fuel cells by 2005.
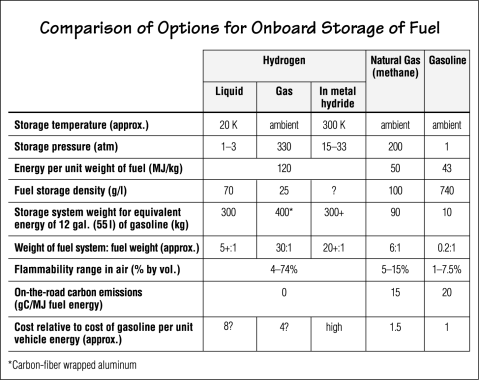
To expedite the switch, Sunline will begin by trucking in liquid hydrogen from a nearby chemical plant and retrofitting or replacing a few buses to use the new fuel. Hydrogen will be stored on the roof of the vehicle in superinsulated storage tanks at slightly above atmospheric pressure and at low temperature (about 20 K) to keep it in liquid form. (See the table below for information about various onboard fuel storage options.) The company will develop special procedures and facilities for refueling the buses, for performing maintenance and repairs, and for operating the buses in a safe manner.
 |
Special handling is required because of the nature of hydrogen. Some of the
stored liquid continuously boils off; so when the bus is idle or shut off, hydrogen
gas accumulates inside the tank. Because molecules of hydrogen are tiny, they
will readily leak through seals in tanks and pipes. But preventing their escape
is critical because hydrogen is flammable over a wide range of concentrations
in the air (see the table). The safest approach is therefore to purge the gas
that forms inside the tank to prevent excessive accumulation. Because hydrogen
is odorless and colorless, hydrogen detectors are required at the fueling station
and on the buses. And knowledgeable, well-trained personnel must perform all
the handling of the hydrogen, including refueling.
Much can be learned from such a demonstration. However, commercial supplies of liquid hydrogen are limited and expensive. Therefore, Sunline plans to manufacture its own hydrogen fuel as soon as it can. As part of the assessment, Ms. Brydges and Dr. Drake reviewed the pros and cons of various approaches Sunline could take.
Again, for an essentially emissions-free vehicle, the hydrogen would have to be derived from renewable energy sources. Solar or wind power, for example, could provide energy to electrolysis machines that would split water into hydrogen and oxygen. Or the hydrogen could be made from traditional fuels, with carbon emissions captured and sequestered. But large technical and economic challenges must be overcome before such operations are feasible at a commercial scale.
Another transitional option is to pump liquid fuels such as gasoline or methanol into the vehicle storage tank and to put on board each vehicle a "reformer"--a chemical reactor that transforms the liquid fuel into hydrogen for the fuel cell and exhausts the carbon as CO2. The infrastructure for producing and distributing gasoline already exists. However, gasoline is a complex mixture; and designing an effective onboard reformer is difficult. Methanol is simpler chemically, and it can be processed more easily and efficiently. But a new infrastructure would have to be established to distribute this new fuel. In addition, reforming either gasoline or methanol to hydrogen gives off almost as much carbon as is saved by using the more efficient fuel cell technology. Net CO2 emissions are therefore about the same as CO2 emissions from burning gasoline or methanol in an advanced internal combustion engine.
Another potential source of hydrogen--natural gas--offers several advantages. Natural gas is plentiful in most parts of North America; and the system of pipelines for transporting it is extensive, safe, and familiar to the public. Natural gas contains few contaminants, and reforming it is an established technology.
In its demonstration, Sunline plans to explore the natural gas option. It will obtain natural gas from the existing pipeline network; reform it to hydrogen gas and compress the hydrogen at a central fueling station; and load the compressed hydrogen into storage tanks onboard the bus. Emissions of local pollutants and CO2 will be about the same as from the current CNG-fueled system; but the emissions will be generated not on the bus but at the central fueling station, where the local emissions can more easily be controlled.
However, storing and moving the hydrogen gas pose problems. Systems and techniques designed for natural gas can be used, but some adaptations must be made. Hydrogen gas is only an eighth as dense as natural gas, so higher compression is needed to store a useful amount of fuel in a tank of a reasonable size. CNG is usually stored at pressures around 200 atmospheres; hydrogen gas would be stored at more than 300 atmospheres. Using hydrogen gas rather than CNG therefore requires better seals, monitors, meters, and other special equipment. The need for well-trained managers and operators is critical. An important question to be addressed is whether a fuel tank of acceptable size and weight can store enough hydrogen gas to power a bus on its daily service run. (Storage of hydrogen gas in metal hydrides has been widely studied but is currently unsuitable for buses because of the weight and cost.)
Summarizing their general findings, the Energy Laboratory researchers identified several potential barriers and concerns, both for a demonstration within five years and for longer-term adoption of the hydrogen fuel cell. One major problem is public acceptance. People are wary of hydrogen. The explosion of the Hindenburg, a hydrogen-filled zeppelin, some 60 years ago created a lingering public perception of hydrogen as a dangerous, flammable, and explosive fuel. In fact, hydrogen does ignite easily. Even static electricity can cause ignition. However, hydrogen also disperses unusually readily, so inadvertent ignition is a problem only in confined spaces. (On the Hindenburg, hydrogen probably did cause ignition; but the subsequent burning was due to the construction materials.) Rigorous testing has led many experts to believe that hydrogen is no more dangerous than any other fuel as long as equipment and procedures suited to its particular characteristics are used. Demonstration programs must convince the public that hydrogen fuel is at least as safe as conventional gasoline is. And preventing any accident or malfunction during the demonstration is crucial, as a single mishap could confirm the public's view of hydrogen as being unacceptably dangerous.
Another significant barrier to the introduction of hydrogen fuel cell buses is cost. Estimates suggest that hydrogen would cost several times more per mile driven than gasoline does. The capital cost of making the change is uncertain, even at the local level. Interested fleet operators would need to obtain financial support through government subsidies or private and public partnerships, some of which are already established.
The ongoing regulatory process is also a concern. Standards already exist for connectors, high-pressure storage tanks, liquid storage tanks, and fuel specifications. But regulators are still working on appropriate building and fire safety codes, schedules for vehicle maintenance and inspection, requirements for training managers and operators, and standards and zoning requirements for siting hydrogen refueling stations and for transporting hydrogen fuel. Until such regulations are in place, companies will have difficulty designing demonstrations that are persuasive for long-term implementation.
In the near term, the focus on hydrogen fuel cells is motivated largely by the need to reduce urban air pollution. However, in the long term, society may have to dramatically reduce carbon emissions. If so, the two likely candidates for transportation fuels are hydrogen and electric power, but only if both are produced from non-carbon sources. Breakthroughs in battery technology or development of methods to charge electric vehicles rapidly on the road might tip the balance toward electricity. But if better storage systems for hydrogen are achieved, hydrogen may be the better bet. Predicting now which route to pursue is difficult, but demonstrations like Sunline's will provide vital insights into the use of hydrogen as a transportation fuel, meanwhile producing some near-term benefits with minimal disruption to society.
Jane E. Brydges received an SM degree in MIT's Technology and Policy Program and an MCP degree in MIT's Department of Urban Studies and Planning in June 2000. She is now a strategist in the Corporate Strategy and Knowledge Development Group at General Motors Corporation in Detroit. Elisabeth M. Drake is associate director for new technologies at the Energy Laboratory. This research was funded as part of a broader study of future road transportation technologies by the Energy Choices Consortium, which includes a group of companies and a foundation. Further information can be found in references.