

 |
 |
|
| A number of self-inactivating lentiviral vectors can be used for transgene delivery and RNAi induction. A list of the vectors is given below. | ||||||||
| Packaging Vectors | Biosafety Notes | |||||||
| LentiLox Vectors | ||||||||
| RNAi Vectors | ||||||||
|
|
||||||||
| Packaging Vectors | ||||||||
| We use the 3rd generation packaging systems for lentiviral production originally published by Dull et al. These vectors include: pMDLg/pRRE (gag/pol elements), pRSV-REV, and pMD.G (env elements). Maps are provided courtesy of the Trono laboratory. Click on the images below for larger images. | ||||||||
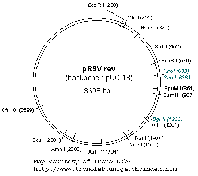 |
||||||||
Sources of these packaging vectors include: Invitrogen- Viral Power Lentiviral
Support Kit K4970-00 |
||||||||
| Biosafety Issues | ||||||||
| IMPORTANT:
The biosafety office at your institution must be notified prior to use
of this system for permission and for further institution-specific instructions.
BL2/(+) conditions should be used at all times when handling the virus.
All decontamination steps should be performed using 70% ethanol/1% SDS.
Gloves should be worn at all times when handling lentiviral preparations,
transfected cells or the combined transfection reagent. Just remember
that although this virus has been significantly modified for biosafety,
it derived from HIV and with a VSV pseudotype human cells can be infected
even if they are not dividing. That said, the
following modifications have been made to prevent viral replication. 1.Packaging
vector lacks both LTRs and has no viral packaging signal (y) 2.The
following viral genes have been deleted from the packaging vector: env,
tat, rev, vpr, vpu, vif and nef. 3.Rev
is supplied in trans on a different vector (RSV-Rev). 4.The
vector expressing the packaged viral genome has a self-inactivating LTR
(TATA box deletion) and expresses no viral gene products. 5.Envelope,
in this case VSVG, is expressed on a separate vector. For
more information please refer to the following papers. Packaging
vectors;pMDLg/pRRE, CMV-VSVG and RSV-Rev): Dull
et al., A Third-Generation Lentivirus Vector with a Conditional Packaging
System. J. Virol. 1998 72(11): 8463-8472. Naldini
L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D.
In vivo gene delivery and stable transduction of nondividing cells
by a lentiviral vector. Science. 1996
Apr 12;272(5259):263-7. Self
inactivating LTR: Miyoshi
H, Blomer U, Takahashi M, Gage FH, Verma IM. Development
of a self-inactivating lentivirus vector. J Virol. 1998 Oct;72(10):8150-7. |
||||||||
| This Webpage was created by Chris Dillon (updated by AED) | ||||||||