Project Amazonia: Characterization - Abiotic - Nutrient Cycles
The Carbon Cycle
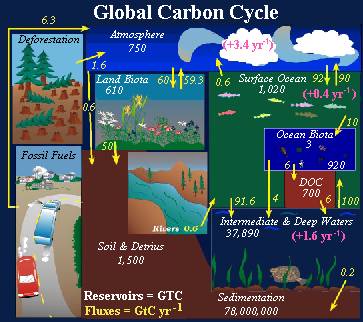
The carbon cycle is the means by which organic wastes can be recycled into practical forms1. Most carbon enters the system as CO2 from the atmosphere, and is then absorbed by plants and converted into carbohydrates through photosynthesis. These carbohydrates are incorporated into the plant matter and released into the soil through decomposition with litterfall. Microorganisms in the soil are responsible for the decomposition of this organic material, producing humic matter and eventually gaseous CO2, which is released into the air, where it can again be absorbed by plants2.
The Nitrogen Cycle
The nitrogen cycle is one of the most important nutrient cycles found in terrestrial ecosystems. Nitrogen is the building block of many complex molecules formed by plants and animals; some examples are amino acids, proteins, and nucleic acids used in DNA.
Most of the world's nitrogen is located in the atmosphere as N2 gas. Plants cannot metabolize atmospheric nitrogen. Plants can only consume nitrogen in two solid forms: the ammonium anion (NH4+) and the nitrate anion (NO3-). However, the former is preferable to the latter because large concentrations of ammonium are extremely toxic.3
In most ecosystems, nitrogen is primarily stored in organic matter. This nitrogen is converted into inorganic forms (forms usable by plants) when it re- enters the biogeochemical cycle via decomposition.3 Decomposition is achieved by various decomposers found in the upper soil layer. They alter the nitrogen found in organic matter into such forms as ammonium salts. This process, known as mineralization, is carried out by a variety of bacteria, actinomycetes, and fungi.3
Animals also require a certain amount of nitrogen in their system for survival. Animals, however, secure their nitrogen fixation through plants or other animals that have fed on plants.
Four processes participate in the cycling of nitrogen through the biosphere: Nitrogen Fixation, Decay, Nitrification, and Denitrification.
Nitrogen Fixation:
Nitrogen fixation is the process by which an organism, frequently a bacterium, transforms a molecule of atmosphere nitrogen (N2), which a plant is not normally capable of using, into a more practical form, usually ammonium (NH4+).
The nitrogen molecule (N2) is inert. It is also stable because of the strength of the triple bond that it possesses. Breaking it apart requires a substantial amount of energy. Most industrial fixation is achieved under great pressure, at temperatures of 600 degrees Celsius, and with the use of a catalyst. Under these conditions, atmospheric nitrogen and hydrogen can be combined to form ammonia. This can be used directly as a fertilizer, but is often further processed into more useful forms such as urea and ammonium nitrate. The ability to fix nitrogen is found only in certain bacteria. The first step in this process produces ammonia. However it is quickly incorporated into protein and other organic nitrogen compounds.
Decay:
When plants die, they decay and leave behind organic forms of nitrogen. This is changed into more useful inorganic forms by certain bacteria.
Nitrification:
Ammonia can be taken up directly by plants- usually through their roots. However, most of the ammonia produced by decay is converted into nitrates. This is accomplished in two steps. Bacteria of the genus Nitrosomonas oxidize ammonia to nitrites. Bacteria of the genus Nitrobacter oxidize nitrites to nitrates. These two groups of bacteria are called nitrifying bacteria.3 Through their activities, nitrogen is made available to the roots of plants.
Denitrification:
The previous processes remove nitrogen from the atmosphere and pass it through the ecosystems. Denitrification reduces nitrates to nitrogen gas, thus replenishing the atmosphere. Denitrification is achieved by bacteria that live deep in the soil and in aquatic sediments. Because the conditions are anaerobic, they use the nitrates as an alternative to oxygen for the final electron acceptor in their respiration.4
Another process, called volatilization turns urea fertilizers and manures on the soil surface into gases that also join the atmosphere, completing the nitrogen cycle.5
Carbon Dioxide
Carbon dioxide is the natural product of many biological and chemical processes, primarily the combustion of organic molecules. Physically, CO2 a non-polar covalent molecule, and therefore has a lower freezing point than water, –78.5ºC. It is relatively uncommon in the air, making up only 0.033% of the atmosphere, but is necessary for the respiration cycles of flora.6
As of present, carbon dioxide represents .033% of the Atmosphere, but over the past 50 years this number has been steadily rising. From 1958 until 2000, carbon dioxide levels in the atmosphere changed from 315 to 375 ppm, a change of 16%, or .4% each year.7 The changing levels of this major greenhouse gas has garnered international attention, with justifiable reason, due to the major implications for all living processes that is plays.
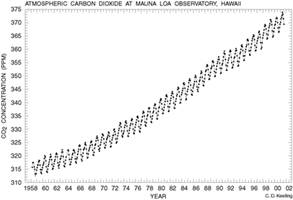
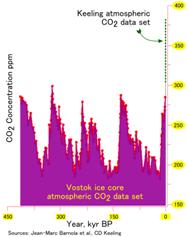
Carbon dioxide is involved in the two of the most important reactions of living organisms: respiration and photosynthesis. In the past, carbon dioxide levels have fluctuated between 175 and 300 ppm in the atmosphere8, but right now the carbon levels are the highest they have been in the past 400 thousand years and are still increasing.
The affects of raised CO2 levels include changes in nutrient absorption, particularly less absorption of nitrogen and sulfur.9 With a decrease of nutrients present in the rainforest trees, all animal species dependent on the tree for food would suffer and become less numerous9. In addition, increased CO2 levels favor early and late successional species in rainforest environments10, causing a change in the forest ecosystem. In addition, raising CO2 levels leans forest ecosystems towards unhealthy monocultures.
The carbon dioxide content change in the glacial ice of Greenland suggests that this increase in carbon dioxide probably began with the start of the industrial revolution. Possible human causes include: The burning of fossil fuels and the clearing and burning of forests, (especially in the tropics). These effects play a significant role in global warming. Carbon dioxide is a good absorber of heat. This phenomenon is known as the greenhouse effect.
Basically, the greenhouse effect is the result of heat absorption by certain gases in the atmospheric and the downward re-radiation of some of this heat. The most abundant greenhouse gas is water vapor, followed by carbon dioxide and other trace gases. An increase in the concentration of greenhouse gases, mainly carbon dioxide, has led to global increases in temperatures.
Rainforests absorb so much of the earth's carbon dioxide that scientists refer to them as carbon sinks. The largest carbon sink in the world today is the ABRE [Amazon Basin Rainforest Ecosystem]. However, deforestation is altering the carbon dioxide absorbing capabilities of the ABRE. Deforestation releases carbon dioxide into the air; it also reduces the amount of trees originally present. These two effects combined result in diminishing carbon sinks which in turn lead to and even more increase in global carbon dioxide concentrations.
These increasing temperatures have a wide array of affects on the rainforest. An article in Nature magazine predicts a dramatic collapse of the Amazonian Rainforest as a consequence of climate chance. Assuming that fossil fuel emissions increase as they are now, the process will begin within a few decades. The cause will be the combined effect of reduced precipitation and increased respiration.
![]()
1: Tan, Kim H., Environmental Soil Science, 2000, Marcel Dekker Inc.
2: NOAA Global Carbon Cycle Program (GCC)
3: Pidwirny, Micheal J. Fundamentals of Physical Geography. Copyright 1996-2002
4: Budyko, M.I. Climate Changes. Waverly Press, Inc. 1977
5: Eltahir, Elfatih A. B. and Bras, Rafael L. Sensitivity of regional climate to deforestation in the Amazon basin. Advances in Water
Resources. 199
6: Morris, C. (editor), Academic Press Dictionary of Science Technology, Academic Press Inc., 1996
7: Numbers based on Mauna Loa observatory measurements
8: Jean-Marc Barnola et al., CD Keeling, High-Resolution Holocene NO2 Ice Core Record and its relationship with CH4 and CO2, Global Biogeochemical Cycles, vol. 16, # 1, 10/29/2001
9: Kanowski, John, Effects of elevated CO2 levels on foliar chemistry of seedlings of two rainforest trees from north east Australia: Implications for folivourous marsupials, Austral Ecology, 26, pg 165-172, 2001.
16: Clark, J.S. et al. Forest succession in CO2 enriched environment Growth Responses of Individuals, Species, and Communities in the Forest-Atmosphere Carbon Transfer and Storage - I (FACTS I) Forest
