

Anthropogenic chemicals have done considerable damage to aquatic ecosystems in the past and continue to do so today. In the U.S., about 220000 miles of 969744 miles of rivers observed in 2009 were deemed to have significantly impaired ecosystem services, due to pollution by chemicals such as heavy metals, especially mercury, pesticides, ammonia, oils and polychlorinated biphenyls (U.S. EPA, 2010). This can be used as an indicator for biodiversity (Grant et al., 2010).
| Size of Water | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rivers and Streams (Miles) | Lakes, Reservoirs, and Ponds (Acres) | Bays and Estuaries (Square Miles) | Coastal Shoreline (Miles) | Ocean and Near Coastal (Square Miles) | Wetlands (Acres) | Great Lakes Shoreline (Miles) | Great Lakes Open Water (Square Miles) | ||
| Good Waters | 448,232 | 5,846,730 | 11,045 | 1,739 | 968 | 208,944 | 78 | 62 | |
| Threatened Waters | 6,369 | 38,681 | 17 | 805 | |||||
| Impaired Waters | 515,143 | 13,081,216 | 21,768 | 7,403 | 307 | 1,101,895 | 4,353 | 53,270 | |
| Total Assessed Waters | 969,744 | 18,966,627 | 32,830 | 9,143 | 1,275 | 1,311,645 | 4,431 | 53,332 | |
| Total Waters | 3,533,205 | 41,666,049 | 87,791 | 58,618 | 54,120 | 107,700,000 | 5,202 | 60,546 | |
| Percent of Waters Assessed | 27.4 | 45.5 | 37.4 | 15.6 | 2.4 | 1.2 | 85.2 | 88.1 | |
The negative effect of these chemicals on organisms is mainly due to their toxicity. The toxicity depends upon the species and environments concerned as well as the properties of the chemical involved (such as the combination of several pollutants or the acidity of the water). The danger posed by a pollutant is therefore hard to gauge (Relya, 2005). Pollution can negatively affect biosystems by the destruction of ecosystems, shifts in ecosystems and bioaccumulation. All of these effects harm the underpinning ecosystems and can significantly impede ecosystem services that are important to us, such as providing potable water, preserving fisheries, protection from floods, and keeping herbivore levels low enough to prevent overconsumption of plant life (Maltby et al., 2010).
Complete destruction of ecosystems occurs when pollutants reach concentrations that are deadly for most of the organisms. This is most often caused by large scale emissions of heavy metals or organic waste. An example of this is demonstrated by a study that involves the river Tame and its tributaries in the English Midlands (conducted by Terry E.L. Langford and others (2010) in "The ecology of industrial pollution"). In pre-industrialized England, the river supported strong communities of fish, invertebrates and aquatic microorganisms. During the industrial revolution, large coal deposits were discovered, which led to the construction of a large steel industry in the area now known as "Black County". After the First World War, this was joined by large coal liquification plants. Both of these industries are very pollution intensive, causing large amounts of heavy metals to be released through atmospheric precipitation and unregulated runoff of ions, acidic waste and ammonia. The pollution from these sources was sufficient to exterminate most fish and sometimes all the organisms in a tributary. It also significantly reduced the number and diversity of organisms in the river Tame itself, rendering the water unsafe for human usage and destroying most fisheries.
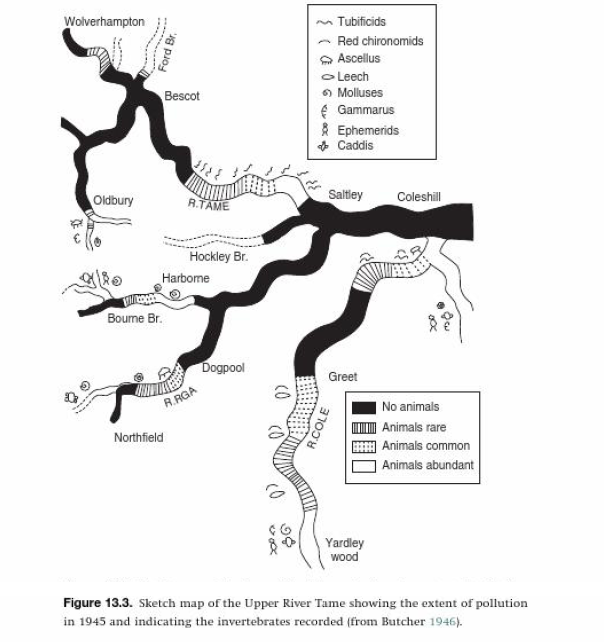 |
In general, this effect can be observed in the misuse of resources. Oil spills, like the "Deepwater Horizon" spill in the Gulf of Mexico, are one example. It can also be observed in the ongoing pollution of the Niger delta and in mine runoffs, especially from gold mines that use heap-leach techniques and in copper ion refuse from copper mines. Untreated industrial waste has also rendered many aquatic environments toxic for organisms and unusable for humans; here, the pollution consists of released organic solvents, polychlorinated biphenyls, dioxins, elusive byproducts (ammonia, chlorohydrogen, sulfuric acid) and acidic waste runoff from the chemical industry, and metal oxides. It also includes minerals, forms of pure metal that coming from smelters or ironworks via runoffs and atmospheric precipitation, and various chemicals used in other industrial branches. During the last 50 years, the amount of chemicals released by private households has also increased strongly – this has mainly evolved due to increased use of soaps, detergents, medicine, plastic, car products and fossil fuels. However, even though the impact of households is growing due to consumption, it is still negligible in comparison to industrial emissions.
Shifts in ecosystems from pollution-intolerant to pollution-tolerant species can be caused by traces of pollutants (Hayes et al., 2002). Some organisms have the ability to ignore them or have better protections than others and are therefore safe while other species go extinct (Grant et al., 2010). While such shifts do not necessarily destroy the ecosystems, they can weaken an ecosystem's resilience and make it less efficient in dealing with other kinds of stress, such as habitat fragmentation and climate change. Also, some ecosystem services may be irreversibly lost or impaired due to the loss of symbionts that results from the loss of a species. This kind of pollution can come from very dissolute sources that are hard to pinpoint ("non-point sources") and include household emissions, industrial wastes, historical toxicant buildups, and agricultural runoff. Shifts can be very taxing on the environment because their effect is usually not confined to one section river or a tributary as is true for destruction. This can lead to overarching regional levels.
One such shift that is a cause for concern is the general decline of amphibian species. Habitat destruction, fragmentation, climate change and other factors contribute to their demise. Many amphibians in relatively protected habitats have seen a significant decline in number and diversity (Blaustein, 1994) . For instance, the abundance of the western toad in the Rocky Mountains had decreased by more than 80 percent (USDI, 2006) from 1970 to 2006. This is at least partly caused by the anthropogenic release of chemicals, according to a study by Tyron Hayes (2002) on the effects of a commonly used herbicide atrazine on amphibians. For African clawed frogs, even very low doses smaller than 0.1 ppb (0.00000001 percent) can cause demasculinization in larvae and thus impair later reproduction. This is an ecologically relevant concentration, as atrazine can reach levels in excess of 40ppb in precipitation and several parts per million in agricultural runoff. In this way, even very low atmospheric concentrations of chemicals used by farms can cause a supranational shift in ecosystems and the demise of amphibians. This extinction may cause significant harm to the ecosystems as amphibians are major insectivores and in turn are eaten by larger predators. Their extinction in an area can lead to excessive levels of insect population as well as declining levels of higher predators.
Bioaccumulation, another way in which pollution can harm the environment, is the internal accumulation of a pollutant that is very difficult to degrade or expel from the body, such as mercury. Every organism accumulates pollutants during its lifetime and if it is eaten, the pollutant are transferred to the predator. Hence, bioaccumulation especially targets the top predators of the food chain, including humans (Meador et al., 1993). In this way, small long-term exposure translates to the accumulation of hazardous concentrations in organisms (Bryan, 1979). Humans are especially endangered through the consumption of fish and other aquatic food, as humans are more susceptible to toxicants like mercury and cadmium than many marine organisms.
One example of bioaccumulation affecting the environment involves a study of pilot whales stranded on Cape Cod in 1986 and 1990. All the whales had elevated levels of mercury in the liver, and the level increased exponentially with age (average level: 176 umol Hg /g tissue mass). Adult whales also had elevated levels of cadmium in their kidneys (average level: 202uml Cd/g tissue mass), though this metal was observed to have an unspecified non-linear correlation with age. All of these heavy metals can cause serious behavioral disorders as well as cancer in mammals and are therefore dangerous under continuous exposure, as was presumably experienced by these pilot whales. The case study also suggested that high levels of mercury and cadmium could be a cause of the increased number of strandings observed.
As evidenced in the case studies given, the different effects of pollution have all reached levels unsustainable for biodiversity. Some are regionally confined, such as most ecosystem destruction cases, but shifts in ecosystems and bioaccumulation can occur on a global scale. They can have overall implications that are just as destructive as the more evident pollutions of oil spills or mine runoffs. Therefore, the solutions to the industrial pollution of aquatic environments need to be as varied and manifold as the actual problem. Solutions must address prevention, treatment and remediation in order to lower the amount of pollutants in the environment as well as its effects.