| home this issue archives editorial board contact us faculty website |

| Vol.
XVI No.
4 February / March 2004 |
| contents |
| Printable Version |
Research at MIT
The Clinical Research Center
In one room a normal volunteer, lying in bed for 12 hours, receives an intravenous infusion of an amino acid labeled with non-radioactive deuterium, part of a study to chart the compound's metabolism in people.
In an adjacent room an HIV-positive subject with disturbed fat metabolism is continuously administered intravenous insulin and glucose, to determine whether his abnormal accumulations of fatty tissues result from inadequate sensitivity to the hormone.
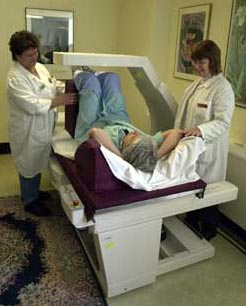
Down the hall a woman with osteoporosis undergoes a scan of her bones to determine whether an experimental treatment really has strengthened them. Later in the day subjects with anorexia nervosa will undergo similar testing.
In a nearby testing room a patient who is recovering from a stroke practices a personalized computer game that may help to restore his ability to make normal hand movements. In one examining room a nurse weighs an obese subject to see whether taking a particular mixture of carbohydrates has made it easier for her to adhere to her weight-loss diet. In another a blood sample is obtained from a young woman who became hypertensive a year ago, while she was pregnant, to determine whether that elevation in blood pressure might have resulted from a persistent low-level inflammation. In one adjacent office two investigators are designing a protocol for testing whether a nutrient normally found in infant formulas can, if given in large doses, repair the memory loss sometimes seen in older people; in another, a professor and his assistant administer a unique training program which teaches young, board-certified physician-specialists how to become clinical investigators.
Perhaps contrary to the reader's expectations, these rooms are not in a Boston-area teaching hospital. Rather, they are on the fourth floor of buildings E17 and E18, within MIT's CRC, or Clinical Research Center.
The CRC was founded in 1962, with major and continuing support from the National Institutes of Health. Its stated goal was to enable MIT investigators to do research on normal subjects and on patients with stable diseases.
Ideally, much of this research would be "translational," determining whether discoveries made in MIT's basic science laboratories also applied to humans, and could yield insights for treating human diseases. (Since MIT's CRC lacked interns or residents, it was unable to take responsibility for studying acutely-ill patients until 2003 when, as described below, it administratively merged with the Massachusetts General Hospital's CRC; now it studies such patients at the MGH.) The CRC admits about 1500 subjects each year, all of whom are outpatients. It finds its research patients by advertising – for example, on the Red Line – or through the hospital associations of its investigators; its normal volunteers most often are MIT students and fellows.
| Back to top |
All of the CRC's costs are covered by its NIH grant, hence the investigator's individual research grant need pay only for the honoraria of some of the subjects; the stipends of students and fellows directly involved in the research; the salaries of staff working solely on the specific CRC project; and – in exceptional cases – the costs of some of the special foods or of biochemical assays. Each year the CRC implements 50-70 active protocols, submitted by 20-30 investigators, involving a wide array of disciplines (e.g., biomedical engineering; neuropharmacology; nutrition-metabolism-endocrinology; psychiatry). All of the protocols have first been approved by the CRC's peer-review Scientific Advisory Committee and by MIT's Institutional Review Board (IRB), The Committee on the Use of Humans as Experimental Subjects (COUHES). All of the subjects receive a full explanation of the project in which they will participate, and sign a consent form. In some studies subjects receive a small honorarium for participating; in others they don't. In any case, their participation is largely altruistic, since the honoraria are not large, and the patients with diseases are clearly informed that treatment of their individual medical problem is not the goal of admitting them to the study.
In fact, the immediate purpose of each study is solely to learn more about a pathological or physiological process.
Before a study can even be considered by the CRC's Scientific Advisory Board it must have been approved by the CRC's resident, NIH-funded statistician (Dr. Mark Vangel) to affirm that the data thus generated will be interpretable. Although the implicit goal of each study is to generate publications in peer-reviewed journals, now and then – rarely – CRC research has led to an actual new treatment for a particular disease, e.g., REDUX for obesity; SARAFEM for severe PMS; melatonin for insomnia.
The day-to-day operations of the CRC are managed by an administrator (Susan Dalton), a nurse-manager (Marguerite Parkman), and a complement of research nurses, bionutrition experts, core laboratory personnel, informatics specialists, and various assistants. This staff is directed by five physician/investigators who constitute the CRC's program direction and who also hold academic appointments (indicated in parentheses) at Harvard Medical School, and staff appointments at the MGH. They are Dr. Lee Schwamm, a stroke specialist (and Associate Professor of Neurology) who serves as the CRC's Associate Director; and three Assistant Directors, Drs. Roger Pitman, a biological psychiatrist and expert on post-traumatic stress disorder (Professor of Psychiatry); Ravi Thadhani, a specialist in hypertension and kidney disease (Assistant Professor of Medicine); and Steven Grinspoon, a neuroendocrinologist who also investigates AIDS-related metabolic disorders (Associate Professor of Medicine). I serve as Program Director, and my own clinical studies relate to neurotransmitters and to endocrinology/nutrition. My main appointment is, of course, at MIT, but I'm also fortunate to hold appointments at Harvard and the MGH – where I took clinical training decades ago.
| Back to top |
Since the CRC is a medical facility, and must thus satisfy state licensing requirements, it is considered to be a component of the MIT Medical Department, directed by Dr. William Kettyle, and obtains its periodic certifications and its license through that department. But the CRC is also an academic entity – offering an undergraduate course in clinical investigation; providing training opportunities for UROPs, graduate students, and fellows; and organizing annual symposia on "hot" topics in clinical research (e.g., "Neuroprotection in the treatment of Stroke," "Insulin Resistance in Disease," "Post-Traumatic Stress Disorder," "Neuroimaging, a Toolbox for Clinical Neuroscience") for the broader MIT community. In its academic activities it is a component of the Harvard-MIT Division of Health Sciences & Technology (HST), directed by Drs. Martha Gray and Joseph Bonventre. Finally, the CRC is an MIT research center and reports in this regard to MIT's Vice President for Research and Associate Provost, Dr. Alice Gast. Direct oversight of how CRC protocols are implemented - to affirm that each is being conducted exactly as it was approved – is provided by the CRC's NIH-funded Research Subjects Advocates, Dr. Laurence Katznelson and Ms. Joyce Saturley. These Advocates and an MGH research pharmacist (John Vetrano) also review all protocols that administer experimental drugs (including "old" drugs being tested for "new" uses). Such protocols are also overseen by individual Data Safety Monitoring Boards, organized through the CRC, and by the FDA (which must approve an IND – Investigational New Drug – application for the compound being tested). These additional levels of oversight have the salutary effect of enabling the Program Director to sleep more soundly at night.
Any CRC activities that involve the expenditure of grant funds, or that impinge on NIH policies, are vigorously monitored by the NIH's Division of Clinical Research, a component of the National Center for Research Resources. This formidable list of friendly watchdogs notwithstanding, the CRC has almost always operated with a minimum of external input - reflecting, one hopes, the expectation of high standards and good common sense.
About five years ago, with the strong encouragement of the NIH, the program staffs of the MIT and MGH CRCs began holding discussions on the possibility that the two institutions might merge administratively. It seemed that the abilities of both centers to implement the studies that their investigator-constituents were proposing could be materially enhanced if, when appropriate, the centers would share resources and personnel.
For example, MIT investigators might thereby become able to implement protocols involving acutely-sick subjects, and their counterparts at the MGH might have greater access to the specialized outpatient facilities of the MIT CRC; to its state-of-the-art metabolic techniques (e.g., for determining insulin sensitivity using euglycemic hyperinsulinemic clamps and nutrient trafficking by stable isotopes); and, more broadly, to the myriad medically-relevant discoveries being made in MIT basic-science laboratories. Moreover, MIT and the MGH were already well along in planning another joint biomedical venture, the Athinoula A. Martinos Center for Functional and Structural Biomedical Imaging, and MIT and Harvard Medical School had been collaborating for years in running the HST program. (Parenthetically, the MIT and MGH CRCs are about to open a small satellite operation at the Martinos Center, in Charlestown, MA; it will, for example, enable investigators to administer drugs to subjects in imaging studies.)
| Back to top |
These discussions led to a formal administrative merger, followed by the successful submission of a single five-year renewal grant application, funded on December 1, 2002, that provides support for both institutions. Even prior to that award the two centers had established a pattern of sharing resources when appropriate: For example, in 1999 MGH CRC funds were used to purchase a DEXA scanning device that is housed within the MIT CRC and operated by MIT personnel. That device now performs about 1600 scans per year, principally to measure body fat content or bone density. In a sense, its operation proved that the MIT-MGH merger could work to everyone's benefit. In the last year, at least 14 new collaborative protocols have been generated for implementation by investigators at both centers.
Although the MIT CRC receives its own subcontract within the NIH grant that it shares with the MGH, NIH policies require that the larger party to such administrative mergers (i.e., the MGH) be identified as the senior partner and the other as a satellite; and that the program director (or directors) of the larger component serve as overall program director. Thus, from the standpoint of the NIH (but not MIT) the merger caused my "demotion" to Associate Program Director, and Lee Schwamm's to Assistant Program Director. The overall Program Directors are Dr. David Nathan, a distinguished diabetologist, and his Co-Director, Dr. Anne Klibanski, an equally-distinguished neuroendocrinologist; both are Professors of Medicine at Harvard. As far as I have been able to tell, this "demotion" has lacked significant consequences, and both the MIT and MGH CRCs continue to function as largely-independent entities, fully implementing the policies of their home institutions.
The CRC is first and foremost a service facility; its reason for existing is to enable MIT and MIT-affiliated researchers to conduct biomedical studies involving human subjects as efficiently as possible. It strives to be user-friendly. If the reader is interested in taking advantage of its resources, the CRC's program and operating staffs will happily provide whatever assistance is necessary. Please visit our Website for more information: http://web.mit.edu/crc/www .
| Back to top | |
| Send your comments |
| home this issue archives editorial board contact us faculty website |