8) Capillarity and Gravity
-Drops may lose their spherical shape under the influence of gravity.
There exists a particular length, denoted κ-1, beyond which
gravity becomes important. It is referred to as the capillary
length. It can be estimated by comparing the Laplace
pressure γ/κ-1 to the hydrostatic pressure ρgκ-1
at a depth κ-1 in a liquid of density ρ submitted to earth's
gravity g=9.8 m/s2. Equating these two pressures defines the capillary
length:
![]()
For water, the capillary length = 2.7 mm
(i) Shape of drops
 |  |  |
Water drops of increasing size on hydrophobized silicon wafer. Gravity causes the largest drops to flatten.
(ii) Capillary rise in tubes
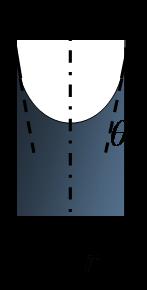
Capillary rise with water: When a narrow tube is brought in contact with a mostly 'wetting' liquid, some of the liquid rises inside the tube. Capillary force supports the weight of the wetting film.
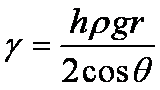
h is the height of the capillary rise with respect to the reference level (height of liquid column in the first tube), g is the acceleration due to gravity (9.8 m/s2), ρ is the density of the liquid, r is the radius of the tube, γ is the surface tension, θ is the contact angle of the liquid on the tube surface
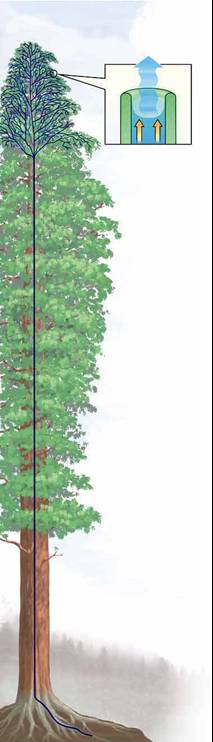
(iii) Capillary forces are responsible for trees growing high!
http://biomechanics.bio.uci.edu/ |
It's certainly a long way up--as much as 100 meters, as
tall as a 30-story building. A tree releases as much as 160 gallons of
water in a day through its leaves and, by necessity, moves that water
up from its roots. How does the redwood manage? Redwoods have a system
of interconnected wood cells for carrying water. The hollow, short, thin
cells are stacked intricately to form an incredibly tall column, extending
from the roots through the branches and stems to the leaves. Water evaporating
from the leaves starts the suction pull. A water molecule evaporates from
a leaf and pulls on the molecules around it as it departs. This creates
a small suction in the water column and pulls water from adjacent water-conducting
leaf cells. These molecules, in turn, attract those around them. (capillary
action)The chain continues to the ground and moves water from the
roots to the tree top just as a pump brings water to the surface from
a well.
Click
here to learn more about the mechanism of rising water inside trees
|
(iv) Capillary profile: curve produced dynamically...
 |
The gap between the two plates increases linearly with the radial distance from the corner. Since, the rise in height is inversely proportional to the gap, we see an 1/x variation with the distance from corner (x).
(v) Shape of drops can give the value of surface tension
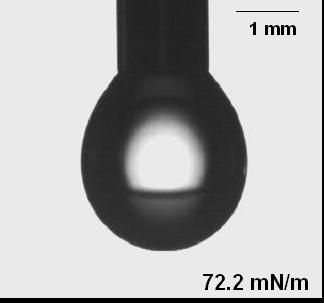
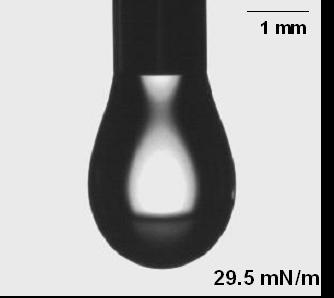
(A) Drop shape with water (B) Drop shape with detergent
The shape adopted by a drop stems from a compromise between the effect of surface tension, which favors a sphere, and gravity, which will cause distortion. For any given situation, analyzing the shape of the drop ought to make it possible to extract the surface tension. This method finds application in lots of commercially available surface tension measurement instruments. Kruss for e.g. makes instruments based on this technology.
(vi) Wet your feet !
 |  |  |
 |
When lid is closed, water does not flow out through the holes
punched at the bottom of the bottle. Surface tension forces are large enough
to balance the gravity forces in absence of external air pressure.
(vii) Water-Hill !

|  |
Glass does not run over when the surface reaches the rim. When a drop of liquid detergent falls and touches the surface of the water hill, the glass immediately runs over. The surface tension, acting like an elastic skin, tries to hold the surface together. Addition of surfactants present in the washing liquid strongly decrease the surface tension.
Back to index ||Introduction to surface tension ||Definition of surface tension||Interfacial tension ||Minimal surfaces ||Soap bubbles, Surfactants and Detergents
Wettability||Role of roughness ||Capillarity and gravity||Dynamical effects and instability||Special interfaces ||Current research in wettability
Back to NIRT Home||On our current research ||More on Wetting||Contact Angle Measurement||Atomic Force Microscopy ||My personal homepage