1) Introduction to Surface Tension
(i) Historical background
Soaps and detergents have been known to mankind for quite a long time now. The earliest known evidence of soap use are Babylonian clay cylinders dating from 2800 BC containing a soap-like substance. A formula for soap consisting of water, alkali and cassia oil was written on a Babylonian clay tablet around 2200 BC. The Ebers papyrus (Egypt, 1550 BC) indicates that ancient Egyptians bathed regularly and combined animal and vegetable oils with alkaline salts to create a soap-like substance. A soap factory with bars of scented soap was found in the ruins of Pompeii (79 AD).Legend has it that soap gets its name from Mount Sapo where ancient Romans sacrificed animals. Rain would send a mix of animal tallow and wood ash down the mountain and into the clay soil on the banks of the Tiber. Eventually, women noticed that it was easier to clean clothes with this "soap". Click here to find more about history of soap making from prehistory days
(ii) Physical origin of surface tension
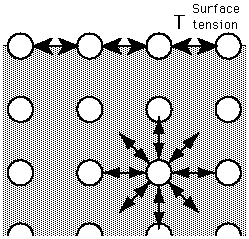
Figure. An 'unhappy' molecule at the surface. It is missing half its attractive interactions, which is why when segregated to the surface, a liquid molecule is in an unfavorable energy state. This is the fundamental reason that liquid adjust their shape in order to expose the smallest possible surface area.
(iii) Practical experiences
-when dry, your hair is likely to be full and thick, whereas the moment it gets wet, it sticks together in a drab, droopy mass.
-early morning, you can see beads of droplets gracing a spider web. The film of dew that has settled on the threads is unstable and breaks up spontaneously into droplets. (this phenomena has in fact implications for textile fibers, process known as 'oiling')
-After stepping out of shower, one dries off by way of evaporation (which can make one feel cold) and by dewetting (the process by which dry areas form spontaneously and expand on one's skin0
- If you ever noticed water drops stuck to windshield, you would know that while large droplets roll down the glass, smallest drops remain stuck.
-No foam is observed in water until you add some additives. (detergents etc.)
-Water spreads on glass, but mercury doesn't stick at all to glass
-While food sticks on stainless steel vessels, it doesn't on teflon vessels.
-Boat powered by surface tension
 |
 |
 |
 |
When the boat is placed gently on the surface of water, it rests on the surface of water suspended by surface tension forces. Upon putting a drop of soap solution/detergent on the notch, boat accelerates rapidly and stops only when it hits the wall. Soap molecules try to spread over the surface of water. Since they are confined in the cavity of boat with only way out, they jet from rear end creating a reaction force which drives the boat forward.
(iv) Commercial importance...
-chemical industry (paints, inks, coloring ingredients, insecticides)
-automobile manufacturing (surface preparation prior to painting, treatment of glass to prevent water from dewetting, treatment of tires to promote adhesion even on wet or icy roadways)
-glass (anti-stain or anti-frost treatment)
-food (dissolving powders such as milk or cocoa)
-soil science (penetration of liquids into porous rocks)
-construction (waterproofing of concrete, protection of monuments, treatment of greenhouse plastic)
-domestics (spreading of creams, application of mascara to eyelashes, self-drying shampoos)
It also plays a role in life sciences.
-rise of sap in plants
-locomotion of insects on the surface of water
-adhesion of parasites on wet surface (e.g., pyriculariosis of rice, or rice blast)
-wetting of the eye. The cornea is by nature very hydrophobic, yet a normal eye is wet ! Proteins (called mucins), present in tears, turn the surface of the eye hydrophilic, stabilizing the lachrymal film. If one accidentally smears a fatty cream on the eye, it dries up, causing considerable discomfort.
Back to index ||Introduction to surface tension ||Definition of surface tension||Interfacial tension ||Minimal surfaces ||Soap bubbles, Surfactants and Detergents
Wettability||Role of roughness ||Capillarity and gravity||Dynamical effects and instability||Special interfaces ||Current research in wettability
Back to NIRT Home||On our current research ||More on Wetting||Contact Angle Measurement||Atomic Force Microscopy ||My personal homepage