Overview. Catalytic synthesis plays a central role in the scalable, economical, and sustainable production of chemicals. Fundamental innovations in both catalyst design and function remain critical to further expanding technical capacity. The overarching objective of our research is to develop new catalysts, strategies, and reagents for synthetic chemistry. The projects we pursue are motivated by: (1) a fundamental mechanistic interest in reaction chemistry, and (2) a practical need for new, efficient, and sustainable synthetic processes. By designing and synthesizing new molecular compounds with unknown structure and function, we hope to learn more about the general principles enabling new chemical transformations.
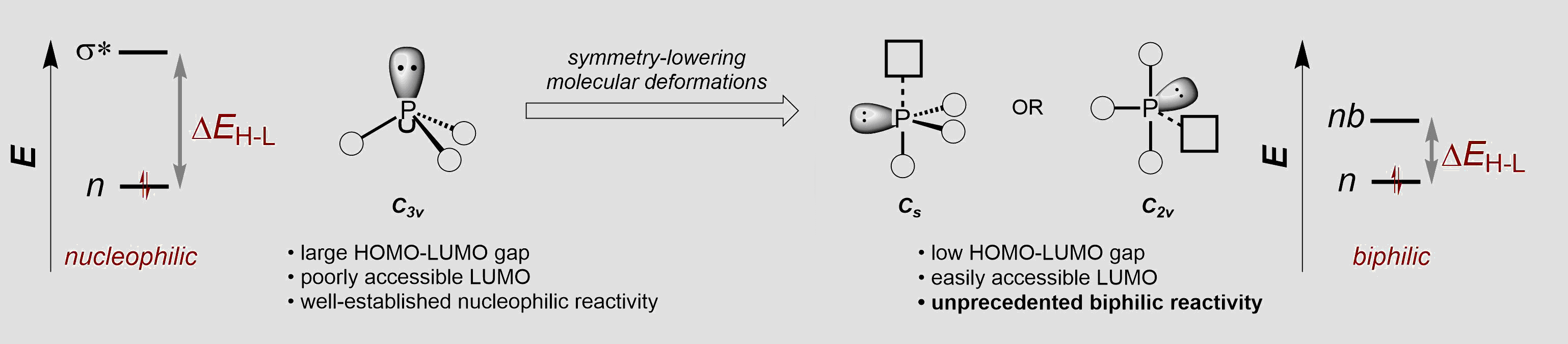
Approach. One recent area of focus has been an exploration of the catalytic potential of phosphorus compounds with unusual low-symmetry molecular geometries. Guided by the precept that molecular geometry dictates electronic structure, we attempt to enforce nontrigonal geometries on tricoordinate P(III) compounds in order to colocalize both electron-donor and -acceptor behavior at a single catalytic site. This approach has enabled the discovery of new ‘biphilic’ organophosphorus compounds that make and break chemical bonds by catalytically cycling in the PIII⇌PV redox couple. Our results represent a departure from the canonical Lewis basic behavior of trivalent phosphorus in catalysis (i.e. as nucleophilic organocatalysts or as supporting ancillary ligands in conventional organotransition metal catalysis).