Subsections
5.2 Axiomatic Statements of the Laws of Thermodynamics
As a further aid in familiarization with the second law of
thermodynamics and the idea of entropy, we draw an analogy with
statements made previously concerning quantities that are closer to
experience. In particular, we wish to present once more the Zeroth
and First Laws of thermodynamics and use the same framework for the
Second Law. In this so-called ``axiomatic formulation,'' the
parallels between the Zeroth, First and Second Laws will be made
explicit.5.1
Section 1.3.2 presented this observation:
Zeroth Law: There exists for every thermodynamic system in
equilibrium a property called temperature. Equality of temperature
is a necessary and sufficient condition for thermal equilibrium.
The Zeroth law thus defines a property (temperature) and
describes its behavior.
Observations also show that for any system there
is a property called the energy. The First Law asserts that
one must associate such a property with every system.
First Law: There exists for every thermodynamic system a
property called the energy. The change of energy of a system is
equal to the mechanical work done on the system in an adiabatic
process. In a non-adiabatic process, the change in energy is equal
to the heat added to the system minus the mechanical work done by
the system.
On the basis of experimental results, therefore, one is led to
assert the existence of two new properties, the temperature and
internal energy, which do not arise in ordinary mechanics. In a
similar way, a further remarkable relationship between heat and
temperature will be established, and a new property, the
entropy, defined. Although this is a much less familiar
property, it is to be stressed that the general approach is quite
like that used to establish the Zeroth and First Laws. A general
principle and a property associated with any system are extracted
from experimental results. Viewed in this way, the entropy should
appear no more mystical than the internal energy. The increase of
entropy in a naturally occurring process is no less real than the
conservation of energy.
Although all natural processes must take
place in accordance with the First Law, the principle of
conservation of energy is, by itself, inadequate for an unambiguous
description of the behavior of a system. Specifically, there is no
mention of the familiar observation that every natural process has
in some sense a preferred direction of action. For example, the flow
of heat occurs naturally from hotter to colder bodies, in the
absence of other influences, but the reverse flow certainly is not
in violation of the First Law. So far as that law is concerned, the
initial and final states are symmetrical in a very important
respect.
The Second Law is essentially different from the First Law; the two
principles are independent and cannot in any sense be deduced from
one another. Thus, the concept of energy is not sufficient, and a
new property must appear. This property can be developed, and the
Second Law introduced, in much the same way as the Zeroth and First
Laws were presented. By examination of certain observational
results, one attempts to extract from experience a law which is
supposed to be general; it is elevated to the position of a
fundamental axiom to be proved or disproved by subsequent
experiments. Within the structure of classical thermodynamics, there
is no proof more fundamental than observations. A statement which
can be adopted as the Second Law of thermodynamics is:
Second Law: There exists for every thermodynamic system in
equilibrium an extensive scalar property called the entropy, 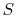 ,
such that in an infinitesimal reversible change of state of the
system,
,
such that in an infinitesimal reversible change of state of the
system, 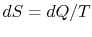 , where
, where 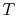 is the absolute temperature and
is the absolute temperature and 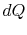 is the amount of heat received by the system. The entropy of a
thermally insulated system cannot decrease and is constant if and
only if all processes are reversible.
is the amount of heat received by the system. The entropy of a
thermally insulated system cannot decrease and is constant if and
only if all processes are reversible.
As with the Zeroth and First Laws, the existence of a new property
is asserted and its behavior is described.
5.2.5 Reversible Processes
In the course of this development, the idea of a completely
reversible process is central, and we can recall the definition, ``a
process is called completely reversible if, after the process has
occurred, both the system and its surroundings can be wholly
restored by any means to their respective initial states'' (first
introduced in Section 1.3.3). Especially, it is to be
noted that the definition does not, in this form, specify that the
reverse path must be identical with the forward path. If the initial
states can be restored by any means whatever, the process is by
definition completely reversible. If the paths are identical, then
one usually calls the process (of the system) reversible, or one may
say that the state of the system follows a reversible path. In this
path (between two equilibrium states 1 and 2), (i) the system passes
through the path followed by the equilibrium states only, and (ii)
the system will take the reversed path 2 to 1 by a simple reversal
of the work done and heat added.
Reversible processes are idealizations not actually encountered.
However, they are clearly useful idealizations. For a process to be
completely reversible, it is necessary that it be quasi-static and
that there be no dissipative influences such as friction and
diffusion. The precise (necessary and sufficient) condition to be
satisfied if a process is to be reversible is the second part of the
Second Law.
The criterion as to whether a process is completely reversible must
be based on the initial and final states. In the form presented
above, the Second Law furnishes a relation between the properties
defining the two states, and thereby shows whether a natural process
connecting the states is possible.
Muddy Points
What happens when all the energy in the universe is uniformly
spread, i.e., entropy at a maximum? (MP 5.3)
Douglas Quattrochi
2006-08-06
|

