|
|
||||
|
Raman Clinical Instrument
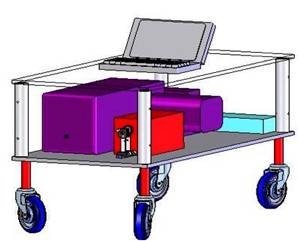
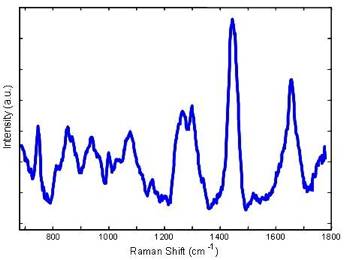
The new Raman system will measure approximately 32” x 17” x 10” and sit on a low wheeled platform. The instrument uses an 830 nm diode laser, delivered through the probe, to excite Raman scattering. The probe delivers to and collects light with the probe tip in contact with tissue. Light from an 830 nm InGaAs diode laser (Process Instruments, Salt Lake City, UT) is passed through a holographic band pass filter centered at 830 nm (Kaiser Optical Systems Incorporated, Ann Arbor, MI). The light is then collimated and coupled into the 200 μm core diameter excitation fiber of the Raman probe. Illumination of the sample is gated by a high-speed, 6 mm aperture, computer-controlled shutter (LS6ZM2, Vincent Associates, Rochester, NY). The excitation fiber is terminated with an FC connector to provide day-to-day reproducibility of alignment. For 100 mW of excitation power, the resultant irradiance is 318 W/cm2 which has been clearly shown to not cause any tissue damage. The proximal end of the probe contains the collection fibers that are arranged in a vertical array and serve as the entrance slit to the spectrograph (Holospec f/1.8i, Kaiser Optical Systems), attached by means of a modified BNC connector. The collected Raman light is dispersed onto a back-illuminated, deep-depletion CCD detector with a 1024×256 array of pixels. The CCD detector is thermoelectrically cooled to -70oC. The probe is 4 m long and is 2 mm in overall diameter. Integrated software (LabVIEW and MATLAB) in the system enables rapid collection of Raman spectra (1s) and real-time analysis of the spectral parameters. Recent Publications
|
||||
| [ Home | Overview | Research | People | Facilities | History | Events | Contact | MIT ] | Lab Intranet |