Spectral diagnosis of cervical dysplasia
The current clinical standard for cervical cancer diagnosis is colposcopy, a procedure that involves visual inspection and biopsy of at-risk tissue, followed by histopathology. Colposcopy is quite sensitive for detection of high-grade squamous intraepithelial lesions (HSIL), which are precancerous lesions with high risk of progression. However, low specificity of colposcopy leads to many unnecessary biopsies, which is invasive to the patient. The aim of our work is to develop a non-invasive clinical tool for detection of cervical HSIL and for guiding the biopsy during colposcopy. We have developed a contact-probe portable instrument for tissue reflectance and fluorescence collection (the FastEEM instrument), and spectral analysis models to extract and quantify biochemical and structural features of tissue to provide disease state assessment. We use a combination of two spectroscopic techniques: Diffuse Reflectance Spectroscopy (DRS), and Intrinsic Fluorescence Spectroscopy (IFS) to analyze tissue spectra.
The clinical in vivo study was conducted at the Boston Medical Center (BMC). The protocol was approved by the BMC Institutional Review Boards, as well as the Committee On the Use of Humans as Experimental Subjects of the Massachusetts Institute of Technology. The contact probe in vivo study involved patients undergoing colposcopic evaluation following an abnormal Pap smear, including atypical squamous cells of undetermined significance (ASC-US), atypical squamous cells- cannot exclude HSIL (ASC-H), LSIL, and HSIL. For each patient, reflectance and fluorescence spectra were collected from a colposcopically normal squamous (CNS) site as well as abnormal sites using the FastEEM instrument. After the application of acetic acid (5% solution) to the cervix during colposcopy, which is a standard clinical procedure, the probe was brought into gentle contact with the tissue. Each measurement which consisted of white light reflectance spectra (300-800 nm emission) and 9 fluorescence spectra (308-460 nm excitation) was acquired in approximately 3s. Colposcopically abnormal sites were then biopsied and evaluated by histopathology. Clinically normal sites were not biopsied.
Each biopsy specimen underwent standard histopathological processing. The hematoxylin and eosin stained tissue sections were evaluated by three experienced pathologists using standard diagnostic criteria. We used consensus diagnosis (agreement of two of the three pathologists) to determine the disease diagnosis of the study samples. Each biopsied site was classified as either negative for SIL, LSIL or HSIL. The spectroscopic parameters of each site were extracted by using DRS and IFS analysis, and were correlated to histopathology diagnosis. The spectral diagnostic algorithm was developed by using logistic regression models based on a combination of significant spectroscopic parameters providing the most important diagnostic information. The schematic representation of the study design is shown in Figure 1.
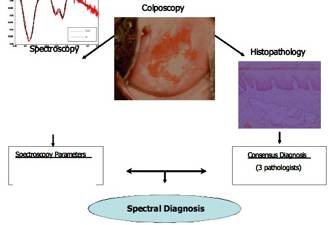 |
Figure 1. Clinical study design. |
The clinical in-vivo study showed cervical anatomy was a confounder to diagnostic algorithms that treat cervix as spectroscopically uniform. We have developed an accurate and site-specific algorithm for detection of HSIL in the cervical transformation zone, an area where vast majority of cervical lesions arise. We further successfully extended our point-probe technique into the imaging mode, to provide the wide-area surveillance capability. The on-going imaging clinical in-vivo feasibility study demonstrates spectroscopic contrast between cervical HSIL and non-HSIL tissue and is consistent with findings of the contact-probe study. The future steps include diagnostic accuracy assessment of the imaging technique, and if proven successful, a clinical study to evaluate the performance of spectroscopy-guided biopsy.
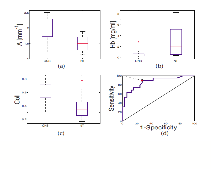
Figure 2. Discrimination of CNS from normal transformation (NT) zone using the contact probe data. Box-plots of (a) A parameter (mm−1); (b) hemoglobin concentration (Hb), (mg/ml); (c) fraction of IFS due to collagen (Coll); (d) LCV ROC plot for the logistic regression algorithm differentiating CNS from NT based on Coll and Hb.
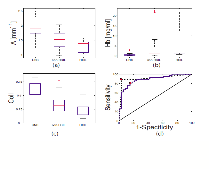
Figure 3. Discrimination of CNS from HSIL and non-HSIL. Box-plots of (a) A parameter (mm−1); (b) hemoglobin concentration (Hb), (mg/ml); (c) fraction of IFS due to collagen (Coll); (d) LCV ROC plot for the logistic regression algorithm differentiating CNS from HSIl sites (dashed line) and CNS sites from non-HSIL sites (solid line).
Recent Publications:
I. Georgakoudi, E. E. Sheets, M. G. Muller, V. Backman, C. P. Crum, K. Badizadegan, R. R. Dasari, and M. S. Feld, “Trimodal spectroscopy for the detection and characterization of cervical precancers in vivo,” Am. J. Obstet. Gynecol. 186(3), 374–382 (2002).
-
J. Mirkovic, C. Lau, S. McGee, C.C. Yu, J. Nazemi, L. Galindo, V. Feng, T. Darragh, A. de Las Morenas, C. Crum, E. Stier, M. S. Feld, K. Badizadegan, “Effect of anatomy on spectroscopic detection of cervical dysplasia,” J Biomed Opt. 14(4):044021 (2009).


|
 |