Spectroscopic diagnosis of oral cancer
Motivation
In the United States, cancers of the oral cavity and oropharynx account for approximately 3% of all malignancies in males, and 2% in females [1]. It is estimated that 35,310 new cases of oral/pharyngeal cancer will be diagnosed in 2008, and 7,590 people will die of this disease [2]. Of these cases, approximately 46% occur in the oral cavity [3]. Early detection is critical for the successful treatment of oral/pharyngeal cancers. The 5-year survival rate falls from 82% to 52%, when the cancer is detected at a distant site, rather than when it is still confined to the primary site (localized) [4]. Unfortunately, only 33% of oral/pharyngeal cancers are localized at the time of diagnosis [4].
Currently the detection of oral cancer relies in large part on the vigilance of a dentist or other health care professional in identifying visible signs of disease. Alterations in surface texture (as from erosion, keratosis, induration, granularity or fissuring), color (red, white, or mixed), and pain may be associated with oral dysplasia or cancer. The presentation can be extremely variable or subtle, however, particularly in the case of early disease. Once a suspicious lesion is identified, a small piece of tissue is cut and removed (biopsy), processed for histology, and examined under a microscope. A pathologist then renders a diagnosis based on a qualitative assessment of the tissue morphology. There are several weaknesses in this detection scheme. First, biopsy is invasive and therefore only a limited number of samples can be taken from the lesion. Second, inter- and intra-observer variability in grading among pathologists limits the accuracy of the final diagnosis.
To overcome these limitations, we use spectroscopy to provide information about the structural and biochemical properties of oral tissue, as these properties may be significantly altered during the disease process. The goal of this project is to use diffuse reflectance spectroscopy (DRS) and intrinsic fluorescence spectroscopy (IFS) to non-invasively and quantitatively identify pre-cancerous or cancerous changes in the oral cavity.
Clinical Studies with the FastEEM
The clinical study was divided into two parts in order to achieve our goal of spectroscopic diagnosis: a healthy volunteer (HV) study and a patient study. The objective of the HV study was to understand the impact of normal anatomy (i.e. buccal mucosa, gingiva, etc.) on the reflectance and fluorescence properties of healthy oral tissue. The objective of the second study was to develop quantitative, spectroscopic diagnostic algorithms for identifying dysplastic/malignant lesions. Study subjects were recruited from Boston Medical Center and the Massachusetts Institute of Technology (HV study only). All data were collected in vivo using the FastEEM point-probe device. Light was delivered to and collected from the tissue using an optical fiber probe. The measured spectra were then analyzed using physical models (DRS and IFS) to extract quantitative parameters. In patients, but not in HVs, a biopsy was performed at the site of data collection. The specimen was then evaluated by 3 pathologists and the consensus diagnosis was considered the final diagnosis.
HV Study
For the healthy volunteer study we analyzed 710 spectra from 79 healthy HVs from nine areas of the oral cavity. The distributions for each spectroscopy parameter were evaluated and compared across all nine sites. We also related the physical parameters to the anatomic features of the site. The major finding of the HV study was that anatomy produces significant spectral variations in the absence of disease. Our data showed that the gingiva and hard palate were considerably different from non-keratinized sites, and that significant variations in the spectral properties existed even among non-keratinized sites. Sensitivities and specificities of >80% could be achieved in separating anatomic sites when only two spectroscopy parameters were combined. Figure 1 shows a binary scatter plot for the buccal mucosa and hard palate clearly demonstrating the significant separation that can occur between anatomic sites in the absence of disease. A model to discriminate between these two sites was developed using logistic regression based on two spectroscopy parameters, the concentration of hemoglobin (cHb) and the A scattering parameter. A sensitivity and specificity of 89% and 95%, respectively, was achieved in separating these two sites.
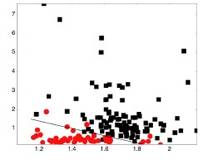 |
Figure 1. Binary scatter plot of the concentration of hemoglobin (cHb) versus the A parameter (scattering parameter extracted from the reflectance) for the buccal mucosa from the hard palate. The diagnostic decision line is also shown. |
From this study, we concluded that anatomy is a significant source of spectral contrast and must be taken into account in the development of spectral algorithms to detect oral pathology.
Patient Study
For the patient study, we analyzed 87 spectra from 64 patients from the same nine anatomic sites as for the HV study. Spectral diagnostic algorithms were developed for the following applications: 1) distinguishing healthy (clinically normal) mucosa from lesions, and 2) distinguishing benign lesions from dysplastic/malignant lesions. For each application, we identified the spectroscopy parameters which provided the most information about the disease status of the tissue, and combined these to generate the spectral diagnostic algorithms. The analysis of the patient data is still in progress.
Future Directions
An endoscopic quantitative spectroscopy imaging (QSI) device, which will permit wide-area imaging of the oral cavity, is currently under development. Using the instrument we will investigate whether spectroscopy has the potential to detect invisible (clinically normal) lesions that are dysplastic/malignant.
Recent Publications
- McGee SA, Mirkovic J, Mardirossian V, Elackattu A, Yu C, Kabani S, Gallagher G, Pistey R, Galindo L, Badizadegan K, Wang Z, Dasari RR, Feld MS, Grillone G. “Model-Based Spectroscopic Analysis of the Oral Cavity: Impact of Anatomy,” J. Biomed. Opt., Vol. 13, 064034 (2008).
- 2. McGee SA, Mardirossian V, Elackattu A, Mirkovic J, Pistey R, Gallagher G, Kabani S, Yu CC, Wang Z, Badizadegan K, Grillone G, Feld MS, “Anatomy-based algorithms for detecting oral cancer using reflectance and fluorescence spectroscopy,” Ann. Otol. Rhinol. Laryngol. 118(11):817-26 (2009).
References
- B. W. Neville and T. A. Day, "Oral cancer and precancerous lesions," CA: A Cancer Journal for Clinicians, 52(4), 195-215 (2002).
- L. A. G. Ries, D. Melbert, M. Krapcho, D. G. Stinchcomb, N. Howlader, M. J. Horner, A. Mariotto, B. A. Miller, E. J. Feuer, S. F. Altekruse, D. R. Lewis, L. Clegg, M. P. Eisner, M. Reichman and B. K. Edwards (eds), "SEER Cancer Statistics Review, 1975-2005," National Cancer Institute, 2008, accessed May 4, 2008.
- B. Rodu and P. Cole, "Oral cavity and pharynx-throat cancer in the United States, 1973-2003," Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology, 104(5), 653-658 (2007).
- A. Jemal, R. Siegel, E. Ward, Y. Hao, J. Xu, T. Murray and M. J. Thun, "Cancer Statistics, 2008," CA: A Cancer Journal for Clinicians, 58(2), 71-96 (2008).


|
 |