Spectroscopic detection of atherosclerotic plaques
Vulnerable plaques are atherosclerotic plaques that are most prone to rupture, which may lead to life-threatening thrombosis. Early detection of these vulnerable plaques is critical in reducing patient mortality associated with cardiovascular disease. Current detection methods, such as angiography, intravascular ultrasound, and magnetic resonance imaging, rely on identifying certain visible morphological features of vulnerable plaques. However, these imaging modalities cannot provide information about the biochemical composition of the arterial tissue, which is essential in accurately identifying vulnerable plaques. Diffuse reflectance (DRS), fluorescence and Raman spectroscopy are distinct spectroscopic modalities that provide different types of information about the target tissue. The combination of these spectroscopic modalities, which is termed multimodal spectroscopy (MMS), can provide biochemical and morphological information that are useful in detecting vulnerable atherosclerotic plaques.
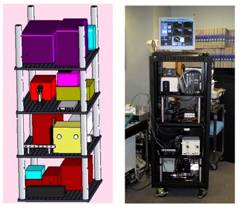
Figure 1. The layout of the MMS instrument (left) and a photo of the actual system (right).
MMS clinical instrument
The MMS clinical instrument consists of an MMS probe, three excitation sources, an optical fiber switch, two separate spectrograph/CCD modules, and a computer. The entire system can be mounted on a wheeled metal enclosure (dimensions 27"×27"×58"), which can be easily transported into and out of the operating room (Figure 1). Prior to surgery, the MMS probe is sterilized by either cold gas ethylene oxide or Sterrad®.
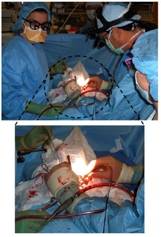
Figure 2. In vivo data collection during a carotid endarterectomy surgery. The surgeon is holding the sterilized MMS probe in contact with the exposed carotid artery plaque, moments before data acquisition. (Courtesy of Arnold Miller, M.D., MetroWest Hospital)
Clinical study
Spectroscopic analysis of human artery tissue with MMS was performed both in vivo and ex vivo, from patients undergoing carotid endarterectomy or femoral bypass (Figure 2). The study was approved by the MetroWest Medical Center Institutional Review Board and the MIT Committee of the Use of Humans as Experimental Subjects. Out of a total of 12 patients, spectra were collected from 40 locations in vivo (9 patients) and 84 locations ex vivo (11 patients). Spectra collection typically consisted of 830 nm, flashlamp, and nitrogen laser exposure with a total exposure time per tissue site of less than 5 seconds. Following spectral acquisition in ex vivo samples, the evaluation site was demarcated with colloidal ink (Figure 3, left) and the specimen was fixed in formalin for histological analysis (Figure 3, right). Based on pathological classification by histological analysis, a spectroscopic algorithm was developed where MMS spectral parameters were extracted to identify key morphological features of vulnerable plaques, such as the presence of large necrotic core, thin fibrous cap, and thrombi. A comparison of this algorithm against histological evaluation indicated that MMS can identify vulnerable atherosclerotic plaques with 89% sensitivity and 78% specificity.
 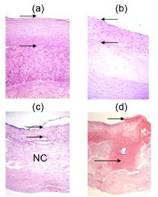
Figure 3. Left : An excised carotid artery plaque with ink dots demarcating spectral evaluation sites. Right : Photomicrographs of tissue sites stained for histological evaluation: a) intimal fibroplasia (H&E; 10X; intimal thickness indicated by arrows); b) thick fibrous cap atheroma (H&E; 10X); c) thin fibrous cap atheroma (H&E; 10X; NC = necrotic core); d) ulcerated plaque with thrombus (short arrow) and intralesional hemorrhage (long arrow) (H&E; 4X)
Research goals
This study aims to develop a spectroscopic analytical tool for identifying vulnerable atherosclerotic plaques, which often evade detection by the imaging modalities currently available in the clinic. We have already shown with open carotid and femoral artery tissues that MMS can identify vulnerable plaques with good sensitivity. We are also investigating ways to develop a minimally-invasive intravascular spectroscopic scanning capability, with the goal of reliably detecting vulnerable plaques in other vital vasculature such as the coronary arteries.
Recent Publications
- O. R. Scepanovic, M. Fitzmaurice, A. Miller, C. R. Kong, Z. Volynskaya, R. R. Dasari, J. R. Kramer, and M. S. Feld, “Multimodal spectroscopy detects features of vulnerable atherosclerotic plaque,” Journal of Biomedical Optics 16(1) (2011).
- O. R. Scepanovic, M. Fitzmaurice, J. A. Gardecki, G. Angheloiu, S. Awasthi, J. T. Motz, J. R., Kramer, R. R. Dasari, and M. S. Feld, “Detection of morphological markers of vulnerable atherosclerotic plaque using multimodal spectroscopy,” Journal of Biomedical Optics 11(2): 021007 (2006).
- J. T. Motz, S. J. Gandhi, O. R. Scepanovic, A. S. Haka, J. R. Kramer, R. R. Dasari, and M. S. Feld, “Real-time Raman system for in vivo disease diagnosis,” Journal of Biomedical Optics 10(3): 031113 (2005).
- J. T. Motz, M. Hunter, L. H. Galindo, J. A. Gardecki, J. R. Kramer, R. R. Dasari, and M. S. Feld, “Optical fiber probe for biomedical Raman spectroscopy,” Applied Optics 43(3): 542–554 (2004).
|
 |