Collaborative Projects
Malaria biology and detection
Malaria remains a major global health problem. With more than 20% of the world’s population at risk, there are about 250 million cases and close to 1 million deaths per year. There are no approved vaccines to prevent the disease, and treatment relies almost exclusively on a limited number of anti-malarial drugs. Parasite resistance to the most efficacious anti-malarial drugs is increasing at an alarming rate. A major factor contributing to the emergence of resistance is the widespread presumptive treatment of symptomatic patients for malaria in the absence of a definitive malaria diagnosis. This decision is often made because easily accessible tools for rapidly, reliably and affordably establishing a definitive malaria diagnosis are unavailable. Thus, developing malaria diagnostic tools that can be used widely in field and clinical settings has enormous potential for more efficiently treating the disease, and preserving the efficacy of the limited number of potent anti-malarial drugs.
In this project, we investigate the interactions of the parasite with red blood cells (RBCs) at the sub-cellular scale and to apply our understanding of malaria biology to develop better diagnostic and therapeutic tools. We are also exploring heme distribution within the parasite under normal conditions and upon perturbation by anti-malarial drugs. In addition, we are evaluating the detection sensitivity and specificity of bulk Raman spectroscopy in diagnosing malaria at different stages of infection.
The Laser Biomedical Research Center has developed unique instrumentation which can measure morphological and chemical information with microscopic resolution. Using the confocal Raman microscope, the chemical composition of RBC (both normal and infected) can be investigated with high spatial resolution. Researchers, including our own laboratory, have shown that hemoglobin and the malaria pigment, hemozoin, have distinct Raman spectra (Figure 1). By quantitative analysis of these Raman signals, different stages can be clearly distinguished. Also anti-malarial drug effects are studied without exogenous staining. Although confocal Raman microscopy provides valuable information regarding RBC shape and its chemical composition, Raman signals are intrinsically weak compared to fluorescence signals. As a result, it is difficult to monitor dynamic events occurring on the sub-minute timescale. Consequently, Raman use in these contexts requires having a fast screening tool to first distinguish infected from normal RBCs prior to intensively acquiring data on the target infected RBCs. In the proposed studies, we will use quantitative phase imaging as the screening tool for rapidly identifying infected RBCs.
Quantitative phase microscopy is a fast full-field imaging technique. Based on interferometry, it provides precise optical path length changes across the imaging plane. The frame-rate of the imaging device, such as CCD or CMOS, is the only factor limiting data acquisition rates. Therefore, it can monitor both static and dynamic information from the sample. This phase information can be converted to sample thickness for RBCs by assuming uniformity of the RBC. Hemozoin generated by the malaria parasite changes the RBC uniformity. Malaria-infected RBCs at different developmental stages can be distinguished by QPM (Figure 2). From static information, shape and refractive index changes in RBC represent infection. Changes in RBC membrane fluctuation also serve as indicators of RBC infection. Although QPM is a fast and full-field imaging technique, it cannot provide the origin of phase delays. As a result, interpretation of QPM images is not trivial. On the other hand, Raman provides abundant information with long acquisition time. By combining the two complementary modalities, malaria biology will be studied from various aspects.
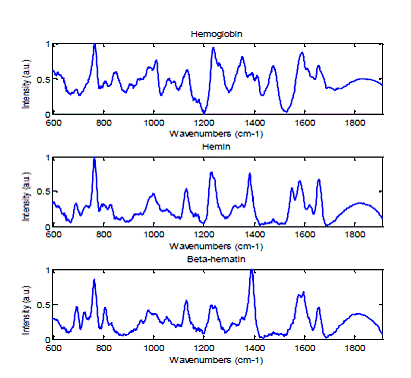
Figure 1. Raman spectra of hemoglobin, hemin and bet-hematin
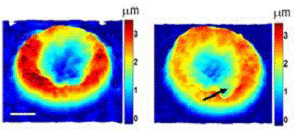
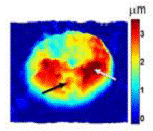 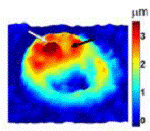
Figure 2.QPM images of malaria-infected RBC with various stages
Relevant Publications
- World malaria report 2009, ISBN 978 92 4 156390 1.
- Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. The Lancet Infectious Diseases 2002; 2:209:218.
- Popescu G, Ikeda T, Dasari RR, and Feld MS. Diffraction phase microscopy for quantifying cell structure and dynamics. Optics Letters 31,775-777 (2006) PMID: 16544620.
- Park YK, Diez-Silva M, Popescu G, Lykotrafitis G, Choi W, Feld MS, and Suresh S. Refractive index maps and membrane dynamics of human red blood cells parasitized by Plasmodium falciparum. Proceedings of National Science of America 2008;105:13730-13735. PMCID: PMC2529332.
|
 |