Collaborative Projects
Spectroscopic monitoring of chemotaxis in microfluidic channels
In this project, we employ Raman spectroscopic techniques to systematically study the metabolic requirements during migration and directional decision making of neutrophils inside microfluidic channels. We study the changes in metabolic consumption of glucose in individual neutrophils moving through straight channels. We establish Raman spectroscopic monitoring in microfluidic channels for the detection of glucose at physiologically relevant concentrations (of the order of 5mM) and compare the concentration changes in front and behind the moving cells. Our studies will create the opportunities for further, more detailed studies in which new technologies are brought together to address clinically relevant questions like wound healing in diabetes or after burn injuries.
In collaboration with, Dr. Daniel Irimia, Assistant Professor, BioMEMS Resource Center at MGH and Harvard Medical School, we are developing several microfluidic devices capable of confining human neutrophils into small channels, for studying their migration in response to chemoattractants. The first generation of devices establishes the chemoattractant gradient along the channels by diffusion between a source and a sink, through the use of well balanced microfluidic streams of distinct fluids. The migration of cells through bifurcating channels are studied using similar devices, helping to quantify the bias in directional decision making in the presence of asymmetric stimulation of cells.
 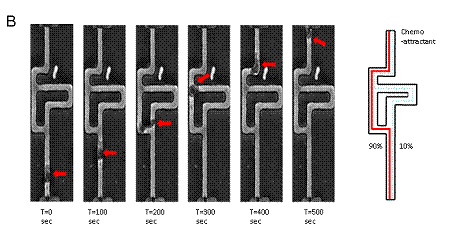
Figure 1. Cell migration in microcapillaries. (A) A linear chemoattractant gradient is present in the empty channels and directs the migration of the HL60 cells (middle channel). After a cell enters one microchannel, a steep concentration difference is established between the front and back of the cell (top and bottom channels). (B) Arrows indicate that the fluorescence intensity is different depending on whether the channel is obstructed or not. Inside asymmetric mazes, human neutrophils chose the shorter path towards the source of fMLP 100 nM in the top channel in 90% of the observations (red outline).
|
 |