Collaborative Projects
Membrane dynamics of human red blood cells
Malaria is a vector-borne disease caused by a protozoan parasite. Every year, it causes the death of several million people worldwide, especially young children in South Africa [1]. When the malaria parasite Plasmodium falciparum (P. falciparum, Pf) invades a red blood cell (RBC), it causes significant mechanical changes to its host. Two major mechanical modifications are the loss of RBC deformability [2-4] and increased cytoadherence of the invaded RBC membrane to vascular endothelium and other RBCs [5]. These changes lead to the sequestration of RBCs in the microvasculature at the later stages of parasite development, which is linked to vital organ dysfunction in severe malaria.
In order to study these mechanical modifications, core researchers at LBRC have investigated membrane fluctuations in Pf-RBCs using diffraction phase microscopy (DPM) [6-7]. Thermally driven membrane fluctuations in Pf-RBCs are strongly correlated with the material properties of cell membranes, which are significantly modified by the specific proteins exported by parasites during developmental stages. Fluctuations in the Pf-RBC’s membrane are used to characterize the membrane stiffness, which is directly related to RBC deformability. The map of instantaneous displacement of cell membrane fluctuation, Dh(x,y,t), was obtained by subtracting time-averaged cell shape from each thickness map in the series. A histogram showing membrane displacement for all parasite stages is shown in Fig. 1 (a), indicating that membrane fluctuations diminish with disease progression.
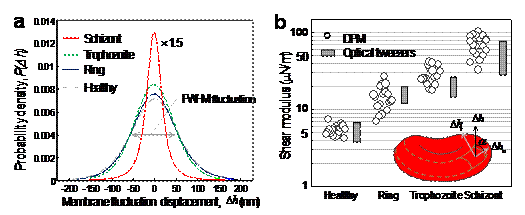
Figure 1. (a) Histograms of cell-thickness fluctuation of Pf-RBCs. (b) In-plane shear modulus of the RBC membrane versus developmental stage of Pf-RBCs. Also shown for comparison are the values estimated using optical tweezers. The measurements were performed at room temperature (23°C) and for each group, 20 samples were tested.
Our DPM experiments provided quantitative information of the membrane fluctuations from which in-plane shear modulus G of RBC could determined using the Fourier-transformed Hamiltonian (strain energy) and equipartition theorem [8]:
where A is the projected diameter of the RBC (~8 mm) and a is the minimum spatial wavelength measured by our DPM setup (~0.5 mm). The tangential component of displacement in membrane fluctuations, Dht2, was decoupled from axial membrane fluctuation Dh2 using the angle a between the direction of Dht and the normal direction of membrane as illustrated in Fig. 1 (b) inset. We have determined that G = 5.5 ± 0.8 μN/m for healthy RBCs, which compares well with independent modulus measurements using micropipette aspiration and optical tweezers approaches. The shear modulus values measured with DPM for the ring (G = 15.3 ± 5.4 μN/m), trophozoite (G = 28.9 ± 8.2 μN/m) and schizont (G = 71.0 ± 20.2 μN/m) stages are in good quantitative agreement with those inferred from large-deformation stretching measurements of Pf-RBCs using optical tweezers, over all stages of parasite maturation [9-10].

Figure 2. Three-dimensional refractive index maps of Pf-RBCs reveal the structural modifications and the hemoglobin concentration of cytoplasm. (A) Healthy RBC. (B) Ring stage. (C) Trophozoite stage. (D) Schizont stage. Images in row show three different cross-sections: 0.6 mm above the focused plane (Top), at the focused plane (Middle), and 0.6 mm below the focused plane (Bottom). Black arrows indicate the location of P. falciparum, and the gray arrows the location of hemozoin. (Scale bar, 1.5 mm.)
In addition to membrane dynamics of malaria-infected RBCs, we have also applied tomographic phase microscopy (TPM) [11] to study the disease states of malaria-infected red blood cells [10]. TPM can quantitatively and noninvasively elucidate the consequences of cell biomechanics of P. falciparum malaria by mapping three-dimensional distributions of refractive index. In Fig. 2, the refractive index maps of Pf-RBCs show the morphological alterations to the host RBCs and the structures of the vacuoles of parasites. In addition, refractive index is translated into quantitative information about the hemoglobin (Hb) content of individual Pf-RBCs. During the intraerythrocytic stages of P. falciparum, we show the decrease of both the total amount and the concentration of Hb in the cytoplasm of Pf-RBCs.
- Galinski MR, Medina CC, Ingravallo P, Barnwell JW. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell. 69, 1213-26 (1992).
- Cranston HA, Boylan CW, Carroll GL, Sutera SP, Williamson JR, Gluzman IY, Krogstad DJ. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science. 223, 400 (1984).
- Nash GB, O'Brien E, Gordon-Smith EC, Dormandy JA. Abnormalities in the mechanical properties of red blood cells caused by Plasmodium falciparum. Blood. 74, 855 (1989).
- Paulitschke M, Nash GB. Membrane rigidity of red blood cells parasitized by different strains of Plasmodium falciparum. The Journal of laboratory and clinical medicine. 122, 581-9 (1993).
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 415, 673-9 (2002).
- Park YK, Popescu G, Badizadegan K, Dasari RR, Feld MS. Diffraction phase and fluorescence microscopy. Optics Express 14, 8263-8 (2006) PMID: 19529201.
- Lue N, Choi W, Badizadegan K, Dasari RR, Feld MS and Popescu G. Confocal diffraction phase microscopy of live cells. Optics Letters 33, 2074-2076 (2008) PMCID: PMC2730468.
- Lee JCM, Discher DE. Deformation-enhanced fluctuations in the red cell skeleton with theoretical relations to elasticity, connectivity, and spectrin unfolding. Biophysical Journal. 81, 3178-92 (2001).
- Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomaterialia. 3, 413-38 (2007).
- Park YK, Diez-Silva M, Popescu G, Lykotrafitis G, Choi WS, Feld MS, Suresh S. Refractive index maps and membrane dynamics of human red blood cells parasitized by Plasmodium falciparum. Proceedings of the National Academy of Sciences of the United States of America. 105, 13730-5 (2008). PMCID: PMC2529332
- Choi W, Fang-Yen C, Badizadegan K, Oh S, Lue N, Dasari RR, Feld MS. Tomographic phase microscopy. Nature methods. 4, 717-9 (2007) PMID: 17694065.
|
 |