MIT Laser Research Facility
Resonance Raman Spectroscopy Applied to Modeling Dioxygen Reactivity the Diiron Center in the Enzyme Methane Monooxygenase
| Investigators: |
Emily C. Carson, Elizabeth M. Nolan, Sungho Yoon and Stephen J. Lippard |
| Department: |
Chemistry, Massachusetts Institute of Technology |
Non-heme iron proteins containing carboxylate-bridged diiron centers perform diverse functions involving transport or enzymatic activation of dioxygen. Among the growing collection of these biomolecules, hemerythrin (Hr) in marine invertebrates binds O2 reversibly for transport to respiratory sites, and soluble methane monooxygenases (sMMO) in methanotrophic bacteria employ O2 for oxidation of a wide range of organic substrates, including the kinetically difficult conversion of methane to methanol. Because of the distinctive reaction chemistry mediated by these proteins, characterization of their structures and reactivity represents an important step toward understanding biological dioxygen activation. These studies may also lead to the generation of synthetic catalysts that mimic these processes for applications in industrial synthesis and environmental remediation.
In our laboratory, we prepare and study small-molecule analogues of the hydroxylase enzyme in sMMO. Specifically, we utilize bulky bridging carboxylate and related ligands to mimic the diiron active sites in the enzyme. Sterically demanding carboxylates facilitate the main synthetic and reactivity objectives by inhibiting undesirable oligomerizations in the self-assembly of dinuclear iron(II) model compounds and in their reactions with O 2 . Resonance Raman spectroscopy at low temperature in fluid solutions of oxygenated synthetic analogs are essential for characterizing reactive intermediates in these processes. These measurements utilize a custom-made glass Dewar capable of surrounding each sample with a dry ice/solvent mixture, an approach that has yielded well-defined spectra for a number of model systems.
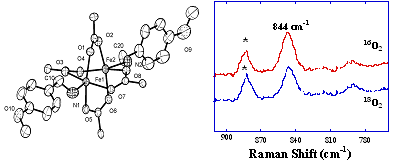
For example, a green intermediate forms upon introduction of dioxygen to a solution of [Fe2(D-O2CArTol)4(BAp-OMe)2] in dichloromethane at -78 oC, where O2CArTol = 2,6-di(p-tolyl)benzoate and BAp-OMe = (MeO)C6H4NH2. This green intermediate has Raman active bands between 800 and 900 cm-1 (Figure 1). Istopic labeling experiments utilizing 18O2 revealed no shift in these bands, which indicates that Fe–O bond formation from reaction of [Fe2(D-O2CArTol)4(BAp-OMe)2 with O2 does not occur. The intermediate was ultimately assigned as a mixed-valent Fe(II)Fe(III) species based on other spectroscopic analyses.
|
| Figure 1. Left: ORTEP diagram of [Fe 2 ( m -O 2 CAr Tol ) 4 (BA p-OMe ) 2 ] with the aromatic rings of Ar Tol CO 2 - removed for clarity. Right: Resonance Raman spectra of intermediates derived from the oxygenation of [Fe 2 ( m -O 2 CAr Tol ) 4 (BA p-OMe ) 2 ] with 16 O 2 and 18 O 2 at -78 o C. |
Recent Publications:
- Yoon S, Lippard SJ, "Water Affects the Stereochemistry and Dioxygen Reactivity of Carboxylate-Rich Diiron(II) Models for the Diiron Centers in OxygenDependent Non-Heme Enzymes," J. Am. Chem. Soc. 127, 8386-8397 (2005).
- Yoon S, Lippard SJ, "Synthesis, Characterization and Dioxygen Reactivity of Tetracarboxylate-Bridge Diiron(II) Complexes with Coordinated Substrates," Inorg. Chem. 42: 8606-8608 (2003).
- Du Bois J, Mizoguchi TJ, Lippard SJ, "Understanding the dioxygen reaction chemistry of diiron proteins through synthetic modeling studies," Coord. Chem. Rev. 200-202: 443-485 (2002). Lippard Lab Website:

|
 |
![]()







