High-Temperature Electrocatalysis
Solid Oxide Fuel Cells and Oxygen Separation Membranes
Concept
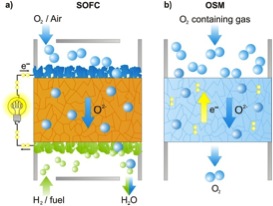
Perovskite oxides have high catalytic activities for oxygen electrocatalysis competitive to platinum at elevated temperatures and thus they are promising candidates for many clean-energy technologies such as solid oxide fuel cells (SOFC) or oxygen separation membranes (OSM). Figure 1 depicts the working principle of both high temperature solid state devices. SOFCs convert chemical energy into electrical energy (Fig. 1a). At the cathode (blue) oxygen is reduced (O2 + 4 e- → 2 O2-), oxygen ions (O2–) move through the solid electrolyte (orange) towards the anode (green) where they react with the fuel (e.g. with hydrogen: 2 O2– + 2 H2 → 2 H2O + 4 e–). The electrons (e–) pass the outer circuit. Comparable processes occur in OSMs (Fig. 1b) oxygen is reduced at one side, oxygen ions pass through the gas tight membrane (other ions cannot pass), and are oxidized at the other side. In contrast to the electrolyte of an SOFC which is a pure oxygen ion conductor (the electrons should pass through the outer circuit), the membrane is composed of a mixed ionic and electronic conductor (MIEC).
Challenges
The main barrier to achieving acceptable chemical-to-electrical conversion efficiency in SOFCs is the sluggish oxygen reduction reaction (ORR) kinetics at the cathode. Similarly, the aim to realize high oxygen fluxes in OSMs requires fast oxygen surface exchange as well as fast oxygen ion diffusion inside the bulk material. Today, a lack of fundamental understanding of the detailed oxygen surface exchange mechanism limits the development of highly active catalysts to enhance the device efficiency. In addition, achieving long term stability of highly active materials at elevated temperature is an important challenge.
Research Highlights
Catalytic Activity Enhancement for Oxygen Reduction on Epitaxial Perovskite Thin Films for Solid-Oxide Fuel Cells and Additional Improvement by Surface Modifications
We study epitaxial thin film perovskite electrodes grown with pulsed laser deposition (PLD) [1-4]. Well-defined electrochemical measurements (e.g. electrochemical impedance spectroscopy (EIS)) are carried out on microelectrodes prepared with photolithography [1-3, 5] (Fig. 2a).
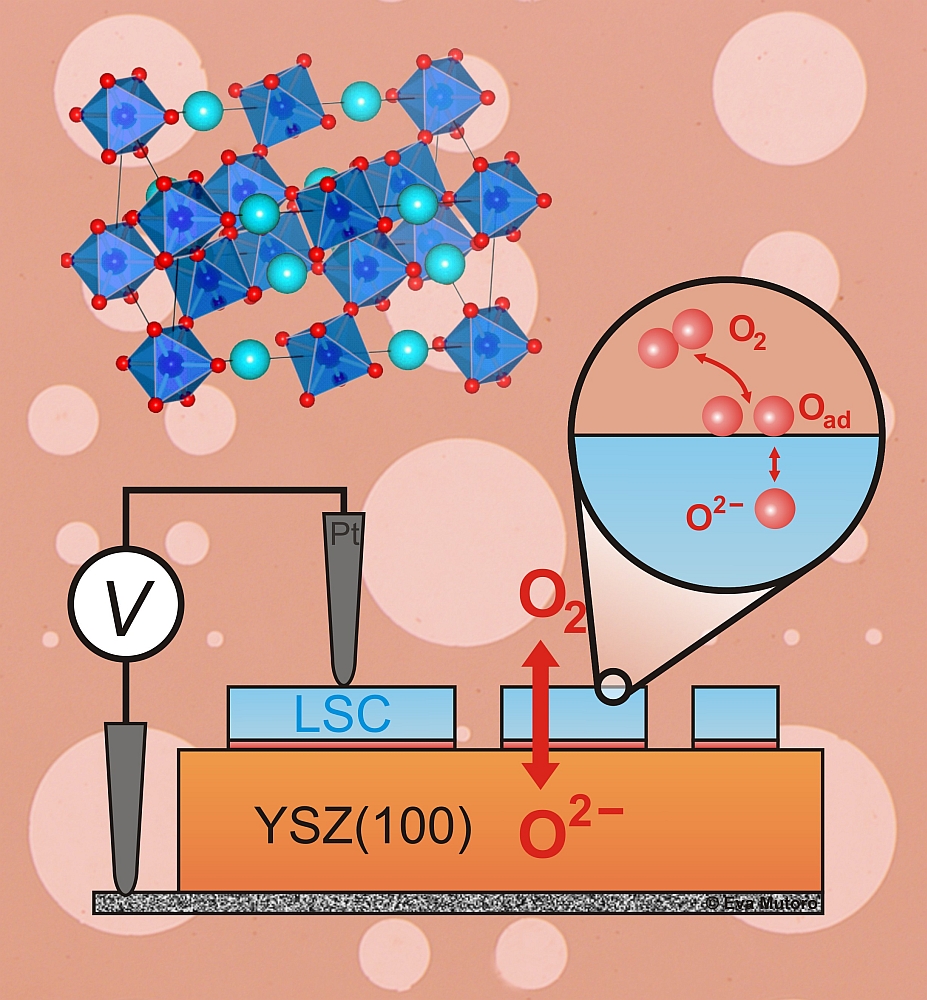
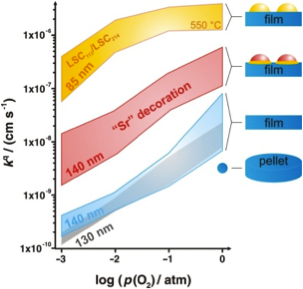
Interestingly, (001) orientated La0.8Sr0.2CoO(3-δ) (LSC113) epitaxial thin films exhibit higher ORR activity than bulk (1–2 orders of magnitude in the oxygen surface exchange coefficient, kq) [1]. The activity can be further enhanced with the decoration of (La0.5Sr0.5)2CoO(4±δ) (LSC214) particles on the surface (overall up to 3–4 orders of magnitude in kq over bulk), where the heterostructured interface between surface LSC113 and LSC214 may contribute to the enhanced ORR activity [2] (Fig. 2b). In addition, the catalytic activity strongly depends on the amount and kind of secondary surface phases, e.g. the catalytic activity of LSC113 surfaces can be modified by decoration with particles composed of Lanthanum-(La) , Strontium-(Sr), and Cobalt-(Co)based (hydr)oxides show different kq values [3].
Reversible Compositional Control of Oxide Surfaces by Electrochemical Potentials
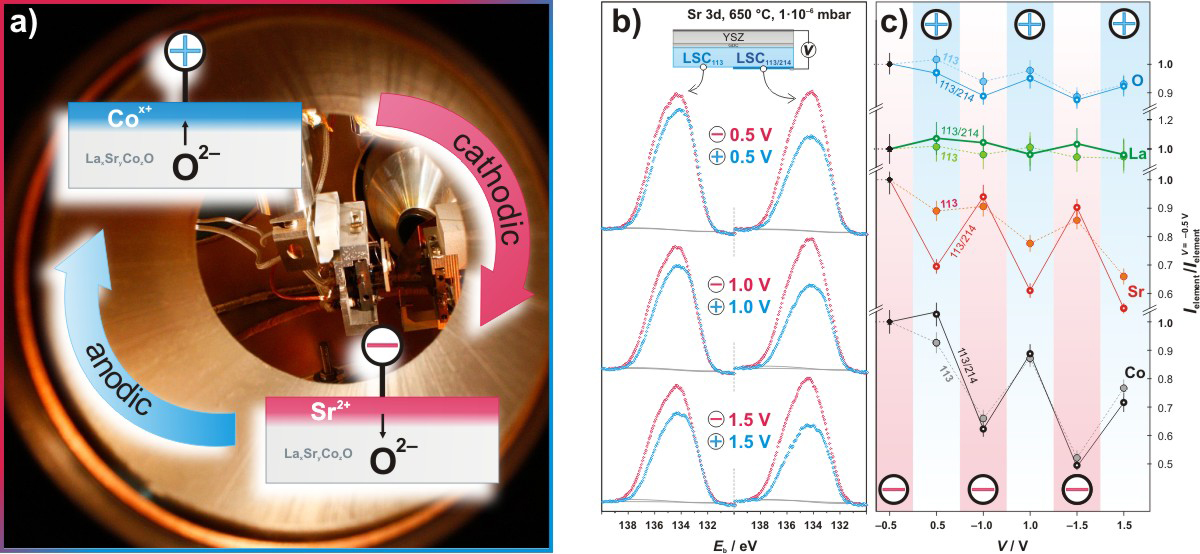
Knowing and controlling the surface composition of perovskite is crucial to enhance device performance. We demonstrated that the surface strontium (Sr) and cobalt (Co) concentrations of perovskite-based thin films can be controlled reversibly at elevated temperatures by applying small electrical potential biases (Fig. 3a)[4]. The surface compositional changes of La0.8Sr0.2CoO(3–δ) (LSC113), La0.5Sr0.5)2CoO(4±δ) (LSC214), and LSC214-decorated LSC113 films (LSC113/214) were investigated in situ by utilizing synchrotron-based X-ray photoelectron spectroscopy (XPS) (Fig. 3b)[4], where the largest changes of surface Sr was found for the LSC113/214 surface (Fig. 3c)[4]. These findings offer the potential of reversibly controlling the surface functionality of perovskites.
Surface Strontium Enrichment on Highly Active Perovskites for Oxygen Electrocatalysis in Solid Oxide Fuel Cells
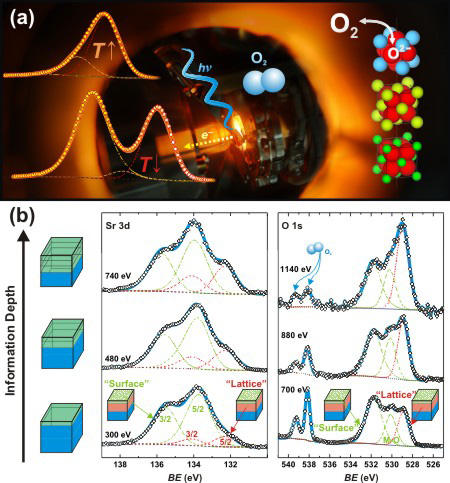
We aim for establishing fundamental principles governing oxide activity for ORR at high temperature. Little is known about the oxide surface chemistry that influences the activity near ambient oxygen partial pressures, which hampers the design of highly active catalysts for many clean-energy technologies such as solid oxide fuel cells. Using in situ synchrotron-based, ambient pressure X-ray photoelectron spectroscopy (Fig. 4a)[6] to study the surface chemistry changes (Fig. 4b)[6], we show that the coverage of surface secondary phases on a (001)-oriented La0.8Sr0.2CoO(3–δ) (LSC) film becomes smaller than that on a LSC powder pellet at elevated temperatures. In addition, strontium (Sr) in the perovskite structure enriches towards the film surface in contrast to the pellet having no detectable changes with increasing temperature. We propose that the ability to reduce surface secondary phases and develop Sr-enriched perovskite surfaces of the LSC film contributes to its enhanced activity for O2 electrocatalysis relative to LSC-powder-based electrodes.
References
- la O’,G. J., S.J. Ahn, E. Crumlin, Y. Orikasa, M.D. Biegalski, H.M. Christen and Y. Shao-Horn, Catalytic activity enhancement for oxygen reduction on epitaxial perovskite thin films for solid oxide fuel cells, Angewandte Chemie International Edition 49 (31) 5344-5347 (July 2010)
- Crumlin, E, E. Mutoro, S. J. Ahn, G. J. la O’, D. Leonard, A. Borisevich, M. Biegalski, H. Christen and Y. Shao-Horn, Oxygen reduction kinetics enhancement on a hetero-structured oxide surface for solid oxide fuel cells, Journal of Physical Chemistry Letters; 1 (21) 3149-3155 (October 2010)
- Mutoro, E., E. J. Crumlin, M. D. Biegalski, H.M. Christen and Y. Shao-Horn Enhanced oxygen reduction activity on surface-decorated perovskite thin films for solid oxide fuel cells, Energy and Environmental Science 4, 3689-3696 (May 2011)
- Mutoro, E., E. J. Crumlin, H. Pöpke, B. Luerssen, M. Amati, M. K. Abyaneh, M. D. Biegalski, H. M. Christen, L. Gregoratti, J. Janek, and Y Shao-Horn Reversible Compositional Control of Oxide Surfaces by Electrochemical Potentials, Journal of Physical Chemistry Letters, 3 (1), 40-44 (January 2012)
- la O’,G. J., R. F. Savinell and Y. Shao-Horn Activity Enhancement of Dense Strontium-Doped Lanthanum Manganite Thin Films under Cathodic Polarization: A Combined AES and XPS Study Journal of the Electrochemical Society 156 (6) B771-B781 (April 2009)
- Crumlin, E. J., E. Mutoro, Z. Liu, M. E. Grass, M. D. Biegalski, Y.-L. Lee, D. Morgan, H. M. Christen, H. Bluhm, and Y Shao-Horn Surface strontium enrichment on highly active perovskites for oxygen electrocatalysis in solid oxide fuel cells Energy and Environmental Science Energy and Environmental Sciences 5 (3) 6081 - 6088 (Jan 2012)

