Low-Temperature Electrocatalysis
Concept
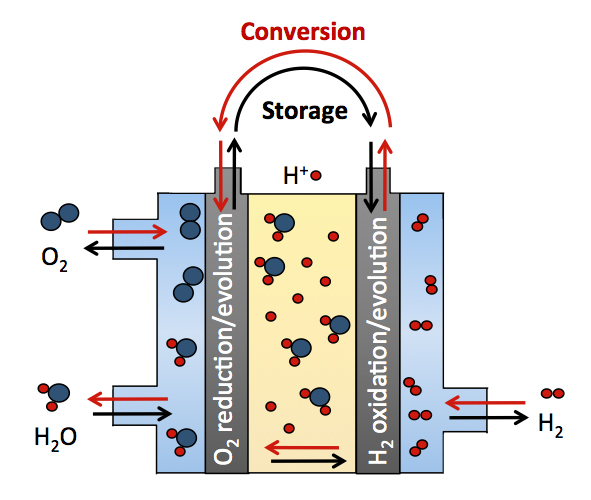
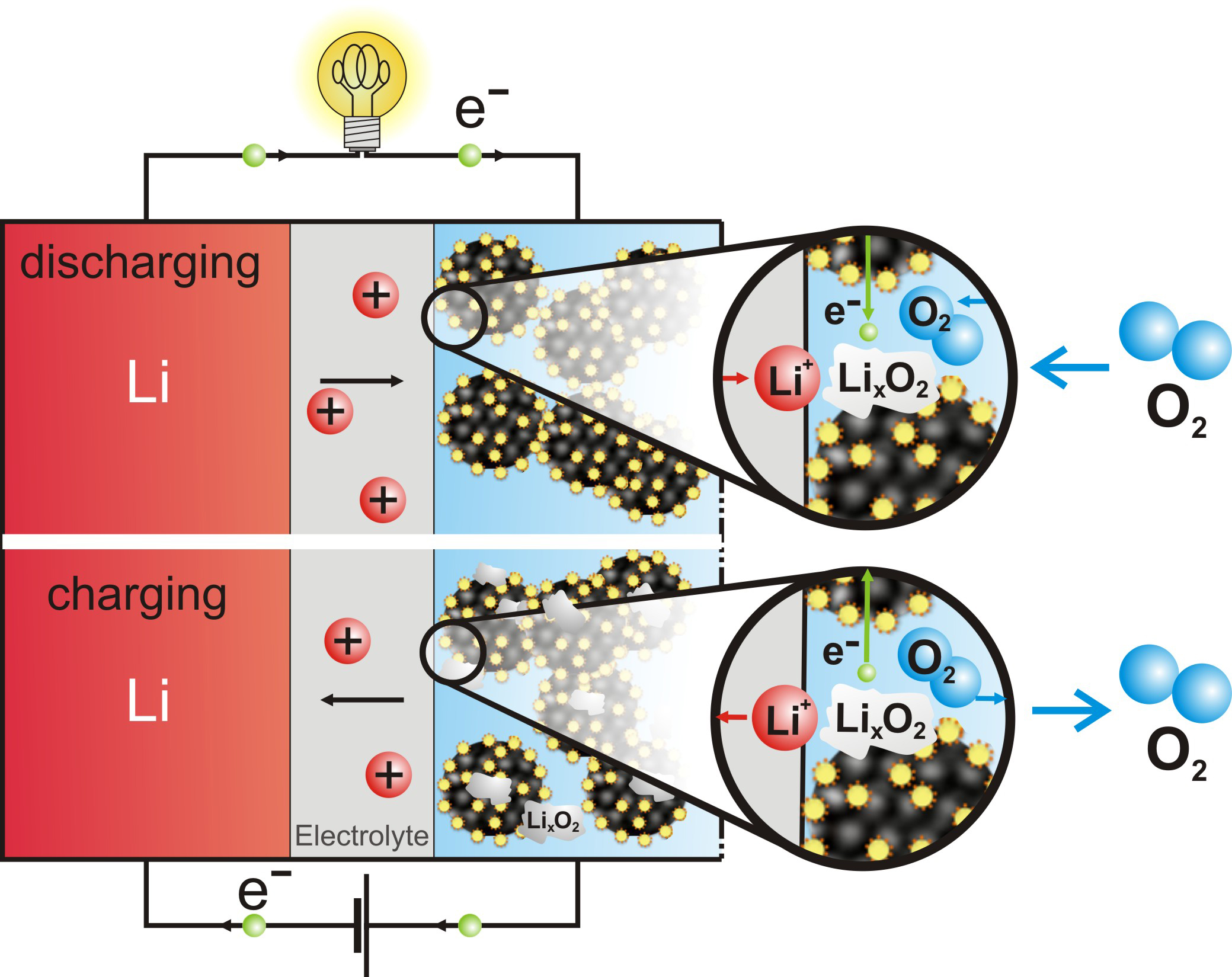
Low temperature electrocatalysis is central to many energy conversion and storage technologies. For example, the energy efficiencies of proton exchange membrane fuel cells (PEMFCs) (Fig 1a), anion exchange membrane fuel cells (AMFCs), and Li-air batteries (Fig 1b). These electrochemical devices directly convert fuels to electricity carried by proton, hydroxide ion or lithium ion at room temperature and rely heavily on oxygen reduction reaction (ORR) and the reverse reaction, oxygen evolution reaction (OER) electrocatalysts. [1]. The energy conversion of these low temperature electrochemical devises is typically less than 70% due to the slow kinetics of the ORR [2, 3] and OER. [4, 5]. The loss of efficiency due to the sluggish electro-kinetics is even further pronounced for direct methanol and other alcohol fuel cells, which also suffers from slow methanol oxidation kinetics in tthe anode in addition to the already sluggish ORR kinetics in the cathode [6].
Challenges
Discovery of cost-effective, stable, highly efficient electrocatalytic surfaces that can catalyze the ORR, the OER, and small organic molecule (e.g. methanol) oxidation can improve the conversion efficiency and is an enabling step for the commercialization of PEMFCs, AMFCs, electrolyzers, and metal-air batteries. A lack of fundamental understanding of the reaction mechanisms and catalyst design principles has been the major hurdle toward better low temperature electrocatalysts.
In addition, novel synthesis methods and characterizations to produce and confirm the formation of the highly efficient structure are needed to create new types of catalyst that are precious metal free. These challenges also apply to the cases of small organic molecule oxidation reaction, where discoveries of new catalysts that can function better and made of cheaper materials are seen as enabling steps toward commercialization of these electrochemical energy technologies.
Research Highlights
The Influence of Surface Chemistry of Metal Nanoparticles on Catalytic Activities
Stability of Metal Nanoparticles in PEMFCs
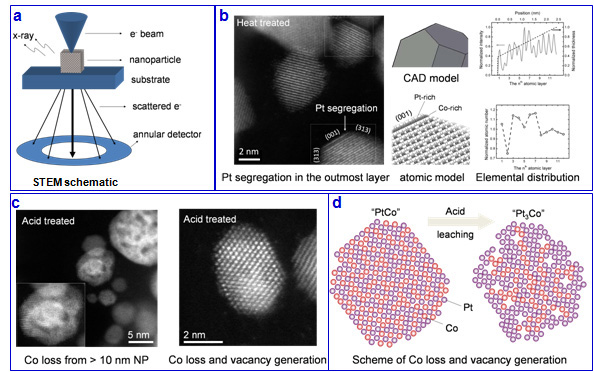
Pt and Pt-alloy nanoparticles are used in the cathode of PEMFCs due to their high activity for ORR. The nanoparticle size, microstructure, and composition affect greatly their catalytic activities. One characterization tool is (Scanning) Transmission Electron Microscopy ((S)TEM) and its related techniques, such as X-ray Energy Spectroscopy and Electron Energy Loss Spectroscopy (Fig 2). With aberration-corrected (S)TEM, we show that percolated structure exist in acid treated “Pt3Co”, and after heat treatment in vacuum, a monolayer of Pt segregation appears , followed by single Co-rich atomic layer (segregation-sandwich structure). The percolated structure and segregation-sandwich structure result in 2 and 4 times enhancement in ORR activity, respectively. [7,8]
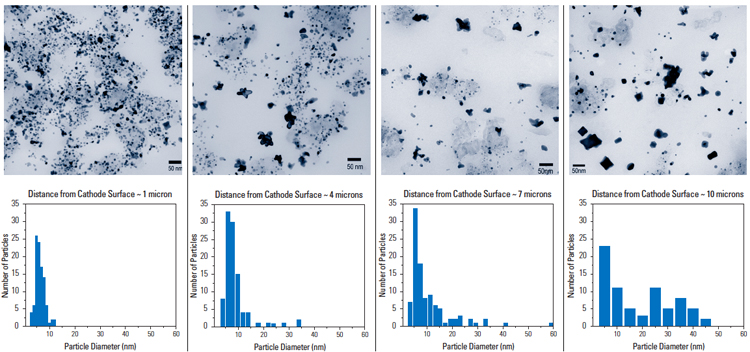
Pt nanoparticles supported on carbon are used as ORR electrocatalysts in fuel cells. Instability of these Pt nanoparticles in PEMFC cathode limits the cell lifetime in automotive and stationary power applications. In collaboration with Prof. Hubert Gasteiger, we have examined cross-sections of fuel cell membrane electrode assemblies in PEMFCs before and after potential cycling. We have found that Pt nanoparticles coarsen significantly by a physical process analogous to Ostwald ripening, and there is a considerable loss of Pt from the cathode as a result of chemical reduction of soluble Ptx+ by H2 near the cathode-membrane interface (Fig 3). Both processes lead to Pt surface area loss and reduced cathode activity. Our studies have clearly suggested that controlling and reducing the solubility of Pt nanoparticles at the cathode is key to maintain cathode activity, particularly upon cell exposure to voltages greater than 0.8 V vs. reversible hydrogen electrode [9]. Ongoing efforts are centered on increasing the stability of surface Pt atoms on nanoparticles against dissolution by increasing particle sizes to around 10 nm and replacing core Pt atoms with oxide nanoparticles to reduce Pt usage.
Design Principles for Oxygen-Reduction Activity on Perovskite Oxide Catalysts For Fuel Cells and Metal-Air Batteries
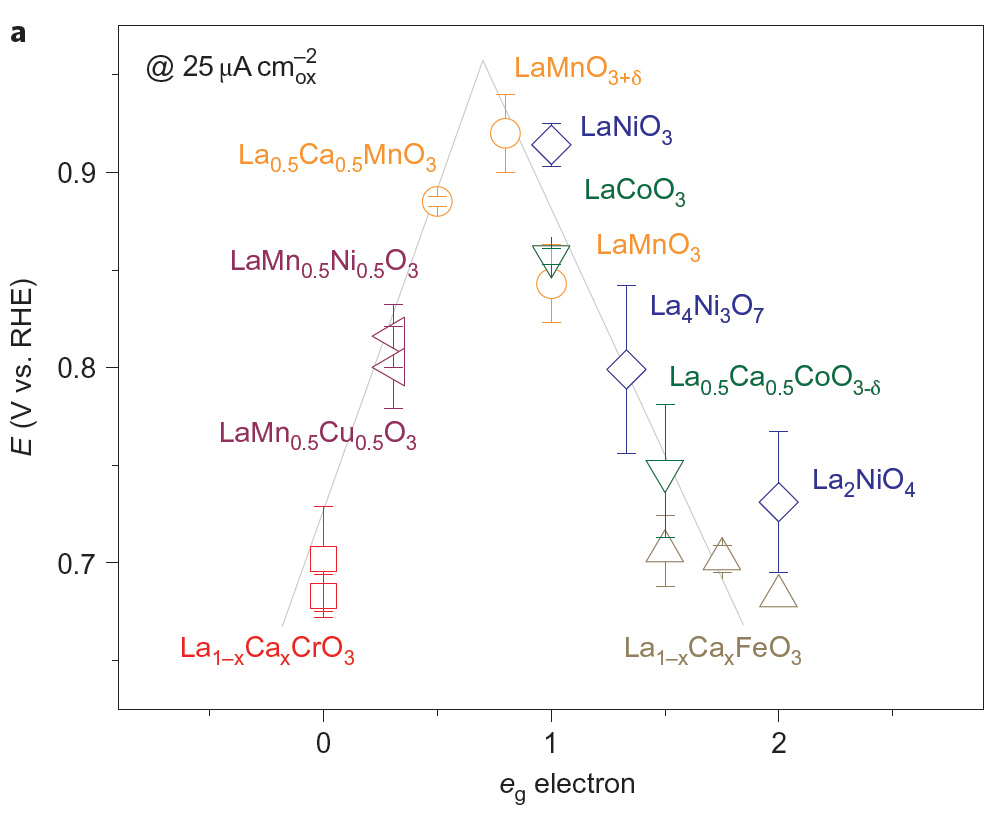
Identifying a precious-metal-free catalyst design principle that links material properties to the catalytic activity can accelerate the search for highly active and abundant catalysts to replace platinum [10]. Although Sabatier’s principle provides a qualitative argument for tuning catalytic activity by varying the bond strength between the catalyst surface and the reactant/product (neither too strong nor too weak, leading to maximum activity at moderate bond strength), it has no predictive power to find catalysts with enhanced activity. We have recently identified unique catalyst properties (“activity descriptors”) that govern the metal-oxygen bond strength and the ORR activity of transition-metal-oxide-based catalysts [11]. Using the oxide-based ORR activity descriptors—the eg orbital occupancy and B-site transition-metal bond covalency, we can quantitatively account for more than four orders of magnitude in tthe ORR activity trend for perovskite transition oxide catalysts (formula: A1–xA'xByB'1–yO3, where A or A' is a rare-earth or alkaline-earth metal and B or B' is a transition metal) (Fig 4). We explain our finding using a molecular orbital framework, which reflects the influences of the eg orbital and metal–oxygen covalency on the competition between O22–/OH– displacement and OH– regeneration on surface transition-metal ions as the rate-limiting steps of the ORR, and thus highlight the importance of electronic structure in controlling oxide catalytic activity.
Catalytic Activity Trends of Oxygen Reduction Reaction for Nonaqueous Li-Air Batteries
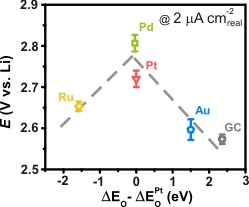
Research efforts toward better understanding of the nonaqueous ORR mechanism and the design principles of highly active catalysts are critical to improve the discharge performance, which directly affects the deliverable gravimetric energy and power of Li-air batteries. We have identified an activity descriptor that governs the nonaqueous ORR activity of metal-based catalysts [12]. We revealed that the nonaqueous Li+-ORR activity of metal surfaces primarily correlates to oxygen adsorption energy, forming a “volcano-type” trend (Fig 5). This volcano dependence suggests that the strength of oxygen binding on the catalyst surface greatly influences the Li+-ORR activity and can serve as a design guide for ORR catalyst for Li-air battery application. In addition, the activity trend found on the polycrystalline bulk surfaces was successfully translated into the discharge voltage of practical Li-O2 cells catalyzed by nanoparticle catalysts.
References
- Gasteiger, H. A.and N. M Markovic Just a dream-or future reality? Science 324 (5923), 48-49 (April 2009)
- Gasteiger, H. A.; S. S. Kocha, B. Sompalli and F. T. Wagner Activity Benchmarks and Rrequirements for Pt, Pt-alloy, and Non-Pt Oxygen Reduction Catalysts for PEMFCs Applied Catalysis B 56 (1-2), 9 (March 2005),
- Piana, M., S. Catanorchi and H. A. Gasteiger Kinetics of Non-Platinum Group Metal Catalysts for the Oxygen Reduction Reaction in Alkaline Medium ECS Transactions, 16 (2), 2045-2055 (October 2008)
- Armand, M. and J. M. Tarascon Building Better Batteries Nature, 451, 652-657 (February 2008)
- Cook, T. R., D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets, and D. G. Nocera Solar Energy Supply and Storage for the Legacy and Non legacy Worlds Chemical Review, 110 (11), 6474-6502 (November 2010).
- Lee, S. W., S. O. Chen, W. C. Sheng, N. Yabuuchi, Y. T. Kim, T. Mitani, E. Vescovo and Y. Shao-Horn, Roles of Surface Steps on Pt Nanoparticles in Electro-oxidation of Carbon Monoxide and Methanol Journal of the American Chemical Society, 131 (43), 15669–15677 (October 2009)
- Chen, S., P. J. Ferreira, W. C. Sheng, N. Yabuuchi, L. F. Allard and Y. Shao-Horn, Enhanced activities for oxygen reduction reaction on “Pt3Co” nanoparticles: Direct evidence of percolated and sandwich-segregation structures Journal of the American Chemical Society 130 (42), 13818–13819 (September 2008)
- Chen, S., W. C. Sheng, N. Yabuuchi, P. J. Ferreira, L. F. Allard and Y. Shao-Horn, Origin of oxygen reduction reaction activity on Pt3Co nanoparticles: atomically resolved chemical compositions and structures Journal of Physical Chemistry C 113 (3), 1109–1125 (December 2008)
- Shao-Horn, Y., W. C. Sheng, S. Chen, P. J. Ferreira, E. F. Holby and D. MorganInstability of supported platinum nanoparticles in low-temperature fuel cells Topics in Catalysis 46 (3-4) 285-305 (November 2007)
- Nørskov, J. K.; T. Bligaard, J. Rossmeisl and C. H. Christensen, Towards the computational design of solid catalysts Nature Chemistry 1 (1), 37-46 (March 2009)
- Suntivich, J., H. A. Gasteiger, N. Yabuuchi, H. Nakanishi, J. B. Goodenough and Y. Shao-Horn, Design principles for oxygen reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries Nature Chemistry 3 (7), 546-550 (June 2011)
- Lu, Y. C., H. A. Gasteiger and Y. Shao-Horn, Catalytic activity trends of oxygen reduction reaction for nonaqueous Li-air batteries Journal of the American Chemical Society 133 (47), pp 19048–19051 (November 2011)

