Photoelectrocatalysis
Concept
One of the major roadblocks to large-scale usage of solar power is the storage of energy during periods of little to no sunlight. One possible solution is the direct conversion of sunlight into chemical fuels, including hydrogen and simple hydrocarbons such as methanol. Figure 1 shows a simplified process where photogenerated charge carriers are used to promote a redox reaction (here water oxidation), thus storing solar energy in the form of chemical bonds. In order to promote this conversion, the electron-hole pair must be separated – typically by an electric field within the device. This places certain requirements on photoelectrode materials; namely, that they exhibit high charge carrier mobility and good light absorption in the solar spectrum.
Challenges
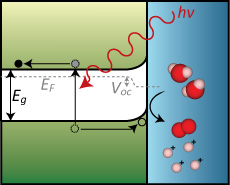
To date, there are two predominant strategies for generating solar fuel via water splitting: (1) “direct” photoelectrocatalysis at the semiconductor-electrolyte interface, i.e. at a solid-liquid junction, and (2) coupling the electrochemical reaction directly to a buried p-n junction solar cell. In the first case, the band edge positions relative to the H+/H2 and O2/H2O redox couples and the band bending at the solid-liquid interface are essential energetic parameters for water splitting. This method should also lead to simpler device architectures. In the second case, existing photovoltaic expertise can be utilized to design a solar cell with the appropriate current-voltage characteristics for solar water splitting. In addition to the photoelectrode requirements of surface stability, good electronic properties and suitable light absorption characteristics, both approaches require the generation of a photovoltage sufficient to split water (> 1.23 V). This is a tall order for any one material, and therefore there are many research fronts for improving solar water splitting devices [1]. In addition to the energetic considerations discussed above, the reactions taking place during water splitting can require large overpotentials to overcome kinetic limitations and proceed at appreciable rates. The use of a co-catalyst on the photoelectrode surface can reduce the overpotential required for a given redox reaction to take place, and in some cases promotes separation and diffusion of carrier species [2,3].
Research Highlights
A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles
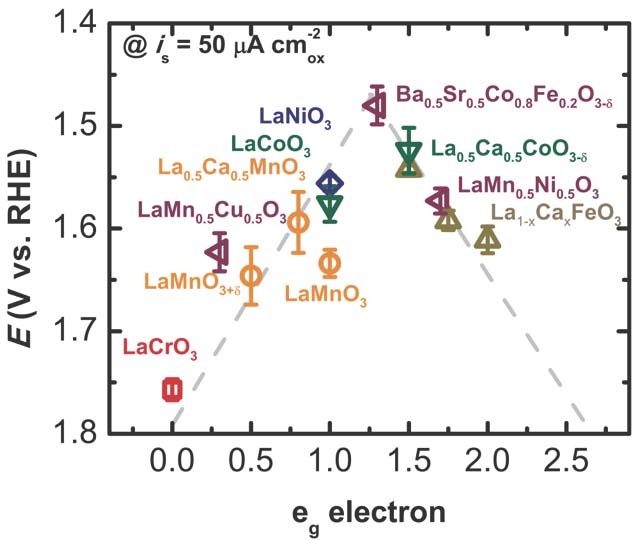
The efficiency of many energy storage technologies, such as rechargeable metal-air batteries and hydrogen production from water splitting, is limited by the slow OER kinetics on the transition metal oxide surfaces. While substantial effort has been devoted to understanding the mechanism and parameters that govern the OER activity and ideally discovering a convenient activity descriptor [4, 5], it is still not straightforward to predict transition metal oxides with high OER activity by means of these previous works. Motivated by the ab initio study of Rossmeisl et al. [6], whose work suggests a universal relationship between the OER activity on the ORR activity, we have recently identified unique catalyst properties (“activity descriptors”) of the OER activity of transition-metal-oxide-based catalysts (namely eg of trqansition metal ions) which can predict a highly efficient OER transition-metal-oxide-based catalyst. Using the eg framework, we predict a highly effective OER catalyst at an eg occupancy close to unity, with high covalency of transition metal–oxygen bond [7]. One candidate that fulfills this criterion is Ba0.5Sr0.5Co0.8Fe(0.2O3-δ), which we found to have a corresponding high OER activity. Our finding reveals that the ORR descriptor can be used for the OER, in agreement with the prediction by Rossmeisl and co-workers [6].
More to come....
There are several areas of research currently under investigation in our lab. We are interested in the discovery and characterization of new photoelectrode materials (with a focus on using in situ methods), as well as the optimization and stabilization of existing candidates such as various oxides and III-V materials. In addition, we are utilizing our previous experience in oxygen evolution catalysis to investigate the integration of co-catalyst materials on silicon photoelectrochemical cells, as well as the properties of the interfaces between co-catalyst, photoelectrode and electrolyte. The overarching goal is a set of design principles derived from a fundamental understanding of the processes and loss mechanisms in these devices.
References
- Walter, M. G. et al., Solar Water Splitting Cells
 Chemical Reviews 110 (11), 6446-6473 (November 2010)
Chemical Reviews 110 (11), 6446-6473 (November 2010) - Meekins, B. H. and P. V. Kamat, Role of Water Oxidation Catalyst IrO2 in Shuttling Photogenerated Holes Across TiO2 Interface Journal of Physical Chemistry Letters, 2 (18), pp 2304–2310 (August 2011)
- Costi, R., A. E. Saunders, E. Elmalem, A. Salant, U. Banin, Visible Light-Induced Charge Retention and Photocatalysis with Hybrid CdSe-Au Nanodumbbells Nano Letters, 8 (2), 637–641 (January 2008)
- Trasatti, S., Electrocatalysis by oxides - attempt at a unifying approach Journal of Electroanalytical Chemistry and Interfacial Electrochemistry (1), 125-131 (July 1980)
- Bockris, J. O. and T. Otagawa, The electrocatalysis of oxygen evolution on perovskites Journal of the Electrochemical Society 131 (2), 290-302
- Rossmeisl, J., Z. W. Qu, H. Zhu, G. J. Kroes, J. K. Nørskov, Electrolysis of water on oxide surfaces Journal of Electroanalytical Chemistry 607(1-2), 83-89 (January 2007)
- Suntivich, J., K. J. May, J. B. Goodenough, H. A. Gasteiger and Y. Shao-Horn, A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles Science 334(6061) 1383-1385 (December 2011)

