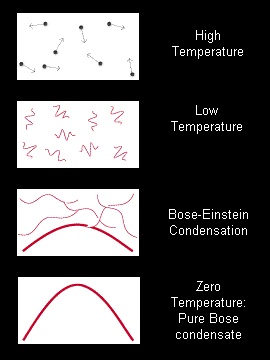
A schematic representation of atoms, shown as waves, condensing into a single quantum mechanical state, the Bose-Einstein condensate.
According to quantum mechanical theory, atoms are not just pointlike particles but also waves. At ordinary (high) temperatures, the wave nature is not as evident as the atom's particle nature. At these temperatures, atoms bounce around like billiard balls (first frame). As atoms are cooled and slow down (second frame), their wave nature becomes evident. As the temperature cools almost to absolute zero (third frame), the waves spread out and, for certain kinds of atoms, begin to overlap. At this point, the atoms lose their separate identities and condense into a single quantum state, the Bose-Einstein condensate. This phase transition has some similarity to the condensation of a gas into a liquid, but is due only to the quantum mechanical wave nature of atoms. As the atoms are chilled to less than a millionth of a degree of absolute zero (fourth frame), almost all atoms join the condensate-a state of matter not known to exist outside the laboratory.
Image from Wolfgang Ketterle, MIT.

